分子别名(Synonym)
CD19,B4,CVID3,MGC12802
表达区间及表达系统(Source)
Human CD19 (20-291), His Tag (CD9-H52H2) is expressed from human 293 cells (HEK293). It contains AA Pro 20 - Lys 291 (Accession # P15391-1).
Predicted N-terminus: Pro 20
Request for sequence
蛋白结构(Molecular Characterization)

This protein carries a polyhistidine tag at the C-terminus.
The protein has a calculated MW of 32.0 kDa. The protein migrates as 43 kDa and 45-55 kDa when calibrated against Star Ribbon Pre-stained Protein Marker under reducing (R) condition (SDS-PAGE) due to glycosylation.
内毒素(Endotoxin)
Less than 1.0 EU per μg by the LAL method.
纯度(Purity)
>90% as determined by SDS-PAGE.
制剂(Formulation)
Lyophilized from 0.22 μm filtered solution in PBS, pH7.4 with trehalose as protectant.
Contact us for customized product form or formulation.
重构方法(Reconstitution)
Please see Certificate of Analysis for specific instructions.
For best performance, we strongly recommend you to follow the reconstitution protocol provided in the CoA.
存储(Storage)
For long term storage, the product should be stored at lyophilized state at -20°C or lower.
Please avoid repeated freeze-thaw cycles.
This product is stable after storage at:
- -20°C to -70°C for 12 months in lyophilized state;
- -70°C for 6 months under sterile conditions after reconstitution.
质量管理控制体系(QMS)
电泳(SDS-PAGE)
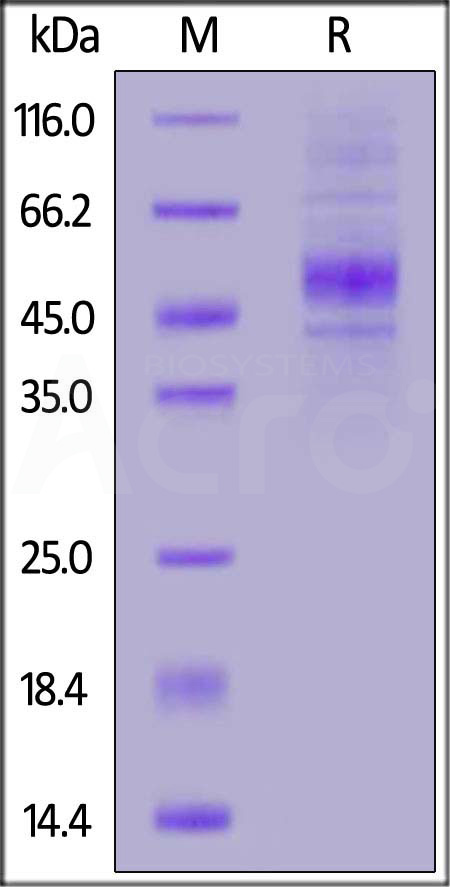
Human CD19 (20-291), His Tag on SDS-PAGE under reducing (R) condition. The gel was stained with Coomassie Blue. The purity of the protein is greater than 90% (With Star Ribbon Pre-stained Protein Marker).
活性(Bioactivity)-ELISA
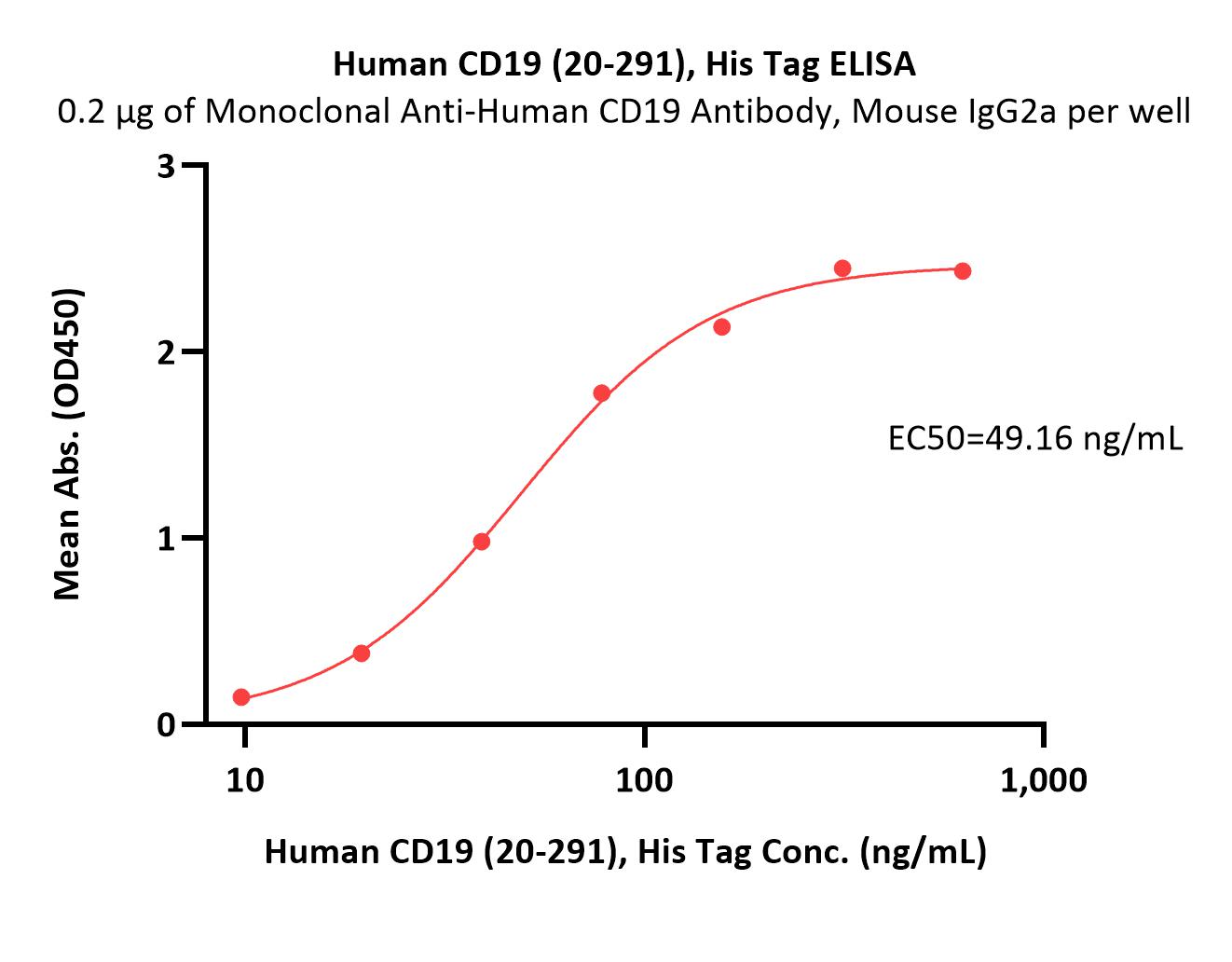
Immobilized Human CD19 (20-291), His Tag (Cat. No. CD9-H52H2) at 2 μg/mL (100 μL/well) can bind Monoclonal Anti-Human CD19 Antibody, Mouse IgG2a with a linear range of 0.2-3 ng/mL (QC tested).
Protocol
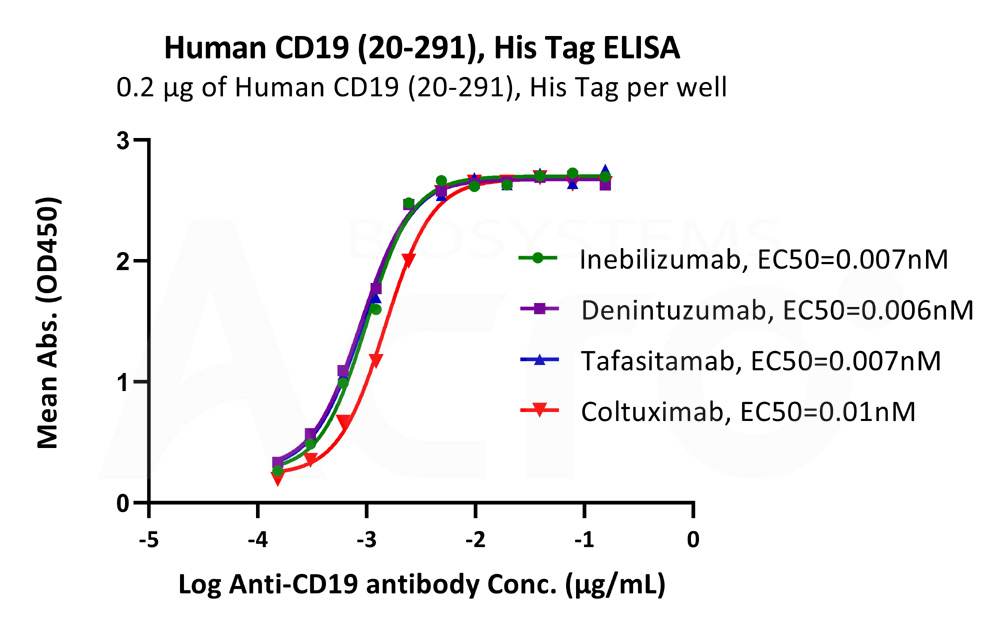
Immobilized Human CD19 (20-291), His Tag (Cat. No. CD9-H52H2) at 2 μg/mL (100 μL/well) can bind different Anti-CD19 antibodies with high affinity (Routinely tested).
Protocol
活性(Bioactivity)-SPR
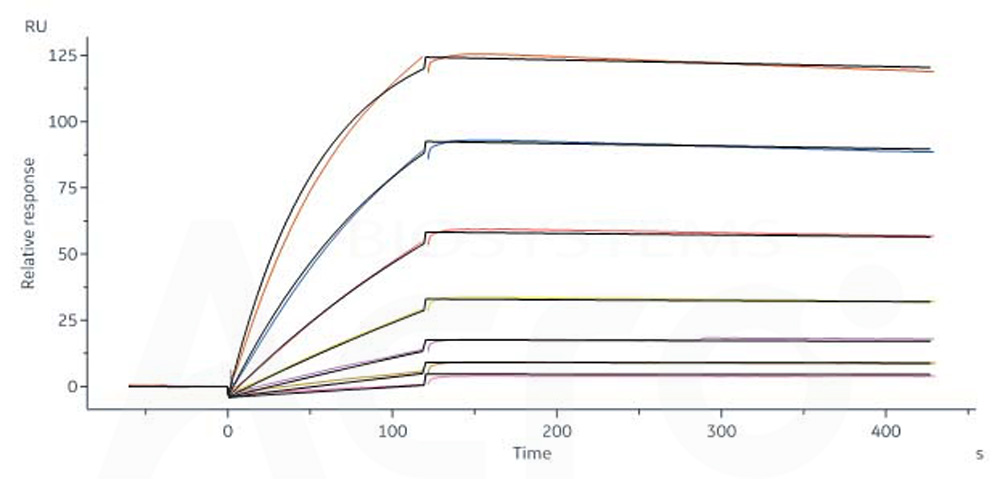
FMC63 MAb (Mouse lgG2a) captured on CM5 chip via anti-mouse antibodies surface can bind Human CD19 (20-291), His Tag (Cat. No. CD9-H52H2) with an affinity constant of 2.9 nM as determined in a SPR assay (Biacore 8K) (QC tested).
Protocol
活性(Bioactivity)-BLI
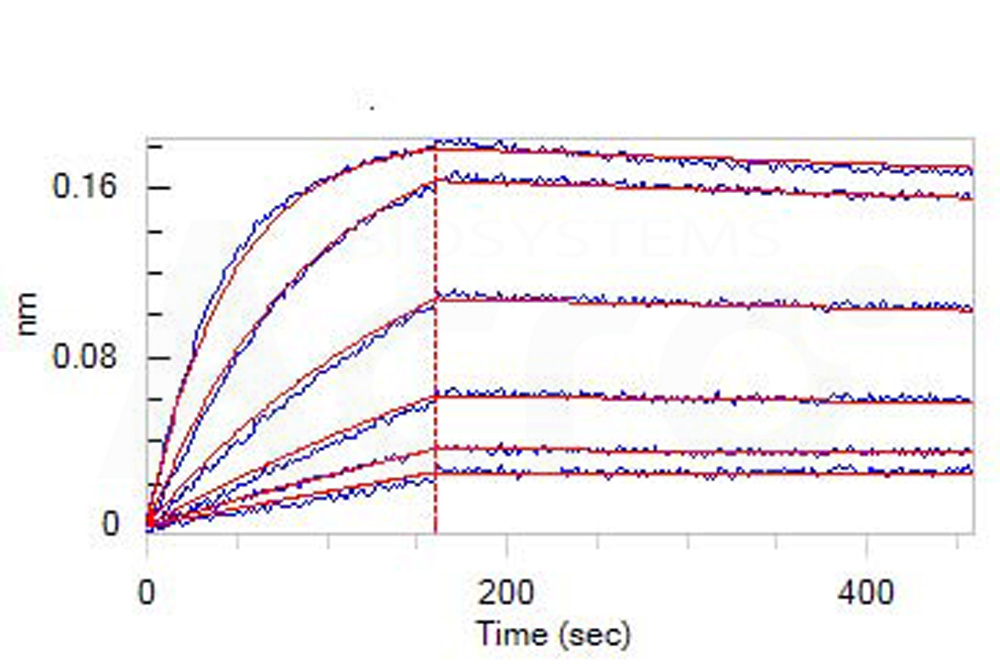
Loaded Monoclonal Anti-Human CD19 Antibody, Mouse IgG2a on AMC Biosensor, can bind Human CD19 (20-291), His Tag (Cat. No. CD9-H52H2) with an affinity constant of 3.19 nM as determined in BLI assay (ForteBio Octet Red96e) (Routinely tested).
Protocol
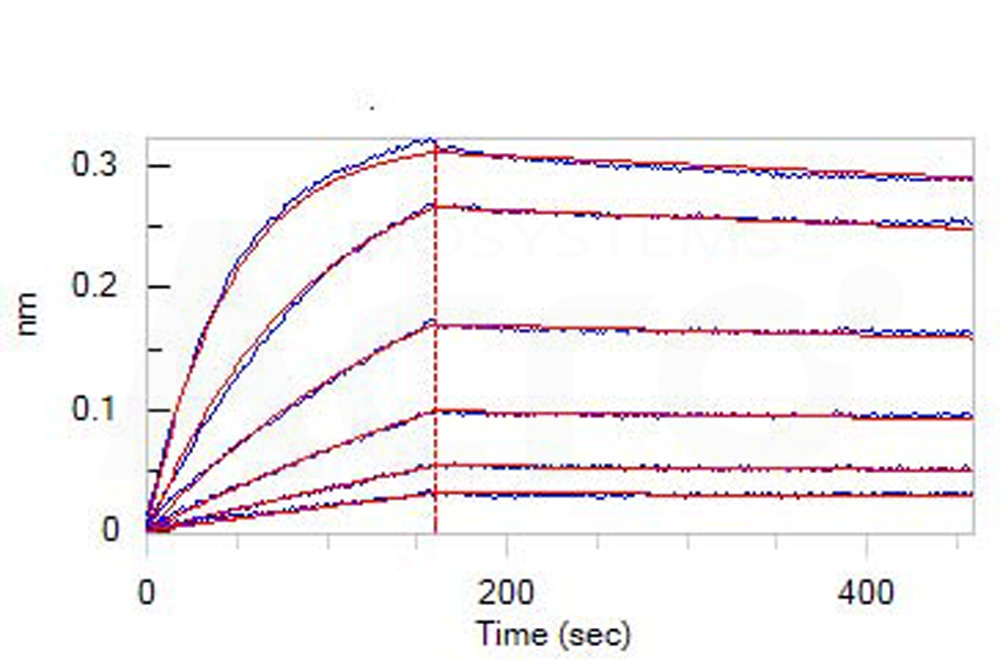
Loaded Monoclonal Anti-Human CD19 Antibody, Human IgG1 on AHC Biosensor, can bind Human CD19 (20-291), His Tag (Cat. No. CD9-H52H2) with an affinity constant of 4.28 nM as determined in BLI assay (ForteBio Octet Red96e) (Routinely tested).
Protocol
活性(Bioactivity)-FACS
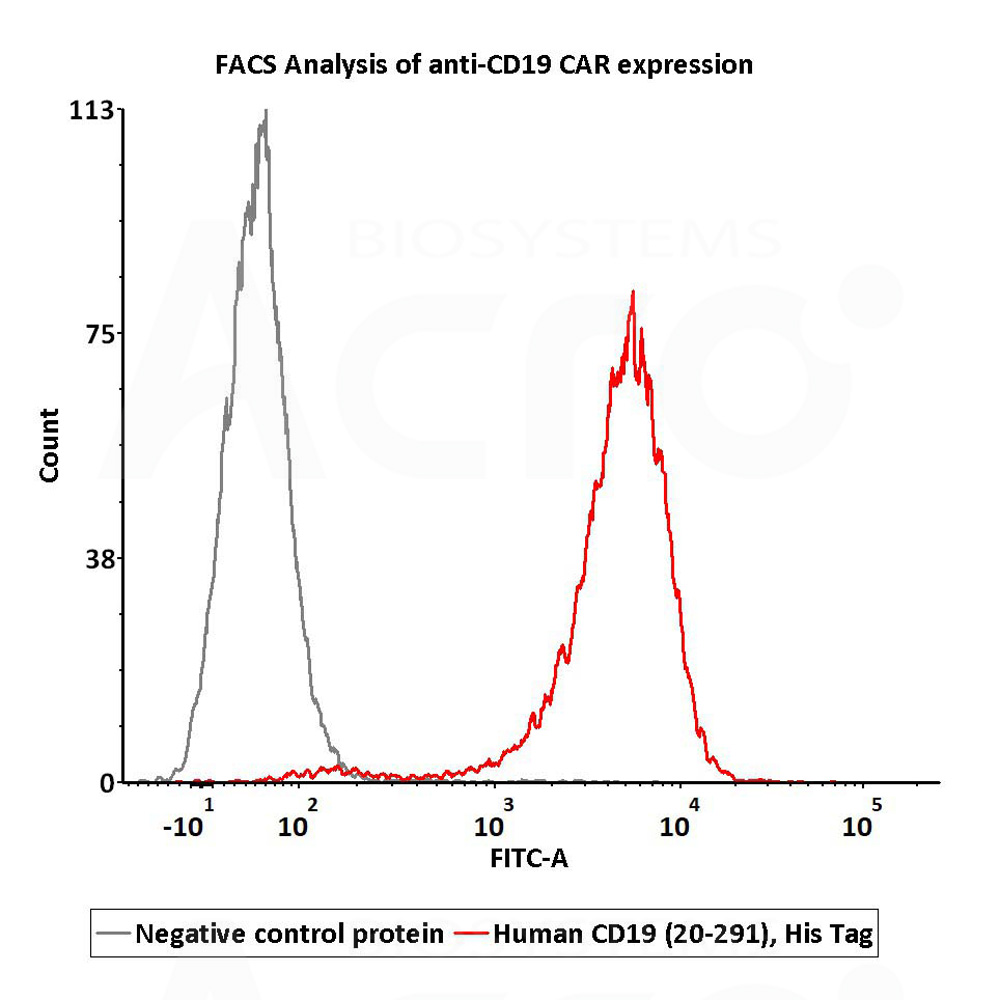
2e5 of anti-CD19 CAR-293 cells were stained with 100 μL of 10 μg/mL of Human CD19 (20-291), His Tag (Cat. No. CD9-H52H2) and negative control protein respectively, washed and then followed by FITC anti-His tag antibody and analyzed with FACS (Routinely tested).
Protocol
 +添加评论
+添加评论背景(Background)
B-lymphocyte antigen CD19 is also known as CD19 (Cluster of Differentiation 19), is a single-pass type I membrane protein which contains two Ig-like C2-type (immunoglobulin-like) domains. CD19 is expressed on follicular dendritic cells and B cells. In fact, it is present on B cells from earliest recognizable B-lineage cells during development to B-cell blasts but is lost on maturation to plasma cells. It primarily acts as a B cell co-receptor in conjunction with CD21 and CD81. Upon activation, the cytoplasmic tail of CD19 becomes phosphorylated, which leads to binding by Src-family kinases and recruitment of PI-3 kinase. As on T cells, several surface molecules form the antigen receptor and form a complex on B lymphocytes. The (almost) B cell-specific CD19 phosphoglycoprotein is one of these molecules. The others are CD21 and CD81. These surface immunoglobulin (sIg)-associated molecules facilitate signal transduction. On living B cells, anti-immunoglobulin antibody mimicking exogenous antigen causes CD19 to bind to sIg and internalize with it. The reverse process has not been demonstrated, suggesting that formation of this receptor complex is antigen-induced. This molecular association has been confirmed by chemical studies. Mutations in CD19 are associated with severe immunodeficiency syndromes characterized by diminished antibody production. CD19 has been shown to interact with: CD81, CD82, Complement receptor 2, and VAV2.























































 膜杰作
膜杰作 Star Staining
Star Staining














