分子别名(Synonym)
TACSTD2,GA733-1,M1S1,TROP2
表达区间及表达系统(Source)
Biotinylated Human TROP-2, His,Avitag (TR2-H82E5) is expressed from human 293 cells (HEK293). It contains AA Gln 31 - Thr 274 (Accession # P09758-1).
Predicted N-terminus: Gln 31
Request for sequence
蛋白结构(Molecular Characterization)

This protein carries a polyhistidine tag at the C-terminus, followed by an Avi tag (Avitag™).
The protein has a calculated MW of 31.2 kDa. The protein migrates as 42-50 kDa when calibrated against Star Ribbon Pre-stained Protein Marker under reducing (R) condition (SDS-PAGE) due to glycosylation.
标记(Labeling)
Biotinylation of this product is performed using Avitag™ technology. Briefly, the single lysine residue in the Avitag is enzymatically labeled with biotin.
蛋白标记度(Protein Ratio)
Passed as determined by the HABA assay / binding ELISA.
内毒素(Endotoxin)
Less than 1.0 EU per μg by the LAL method.
纯度(Purity)
>90% as determined by SDS-PAGE.
>90% as determined by SEC-MALS.
制剂(Formulation)
Lyophilized from 0.22 μm filtered solution in PBS, pH7.4 with trehalose as protectant.
Contact us for customized product form or formulation.
重构方法(Reconstitution)
Please see Certificate of Analysis for specific instructions.
For best performance, we strongly recommend you to follow the reconstitution protocol provided in the CoA.
存储(Storage)
For long term storage, the product should be stored at lyophilized state at -20°C or lower.
Please avoid repeated freeze-thaw cycles.
This product is stable after storage at:
- -20°C to -70°C for 12 months in lyophilized state;
- -70°C for 3 months under sterile conditions after reconstitution.
质量管理控制体系(QMS)
电泳(SDS-PAGE)

Biotinylated Human TROP-2, His,Avitag on SDS-PAGE under reducing (R) condition. The gel was stained with Coomassie Blue. The purity of the protein is greater than 90% (With Star Ribbon Pre-stained Protein Marker).
SEC-MALS

The purity of Biotinylated Human TROP-2, His,Avitag (Cat. No. TR2-H82E5) is more than 90% and the molecular weight of this protein is around 32-47 kDa verified by SEC-MALS.
Report
活性(Bioactivity)-ELISA
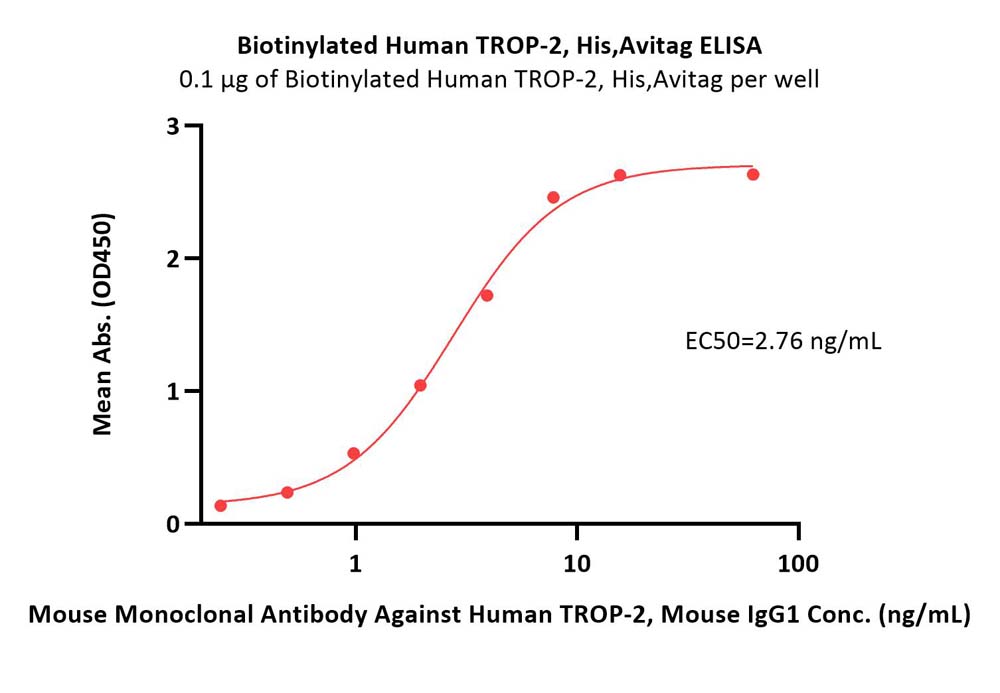
Immobilized Biotinylated Human TROP-2, His,Avitag (Cat. No. TR2-H82E5) at 1 μg/mL (100 μL/well) on streptavidin (Cat. No. STN-N5116) precoated (0.5 μg/well) plate can bind Mouse Monoclonal Antibody Against Human TROP-2, Mouse IgG1 with a linear range of 0.5-4 ng/mL (QC tested).
Protocol
活性(Bioactivity)-SPR

Captured Trop2 antibody on CM5 chip via anti-mouse antibodies surface can bind Biotinylated Human TROP-2, His,Avitag (Cat. No. TR2-H82E5) with an affinity constant of 6.35 nM as determined in a SPR assay (Biacore T200) (Routinely tested).
Protocol
 +添加评论
+添加评论
- 176XXXXXXX0
- 从技术服务知道了这家公司,我们在多项目上委托了BLI服务,服务体验很好,数据及报告做的很漂亮。后续就从百普赛斯直接购买了蛋白,实验数据稳定,蛋白纯度及实验响应比较出色,供货稳定。成为了我们的关键试剂。
- 2022-9-5

- 156XXXXXXX8
- 我们采购该蛋白用于检测自主开发的抗TROP-2抗体的亲和力,通过OCTET检测其亲和动力学,我们用已经获批的IMMU132进行了测试,其亲和动力学常数为nM级别(1.05nM)。
- 2022-4-6
背景(Background)
TROP-2 is a single-copy gene in human cells, and encodes a type-1 transmembrane glycoprotein which is over-expressed in various malignancies, also referred to as tumor associated calcium signal transducer 2 (TACSTD2), GA733-1 or M1S1. TROP-2 is related to epithelial cell adhesion molecule (EpCAM), also called TROP-1, gp40, and KSA. Trop-1 and Trop-2 are homologous to serum IGF-II-binding proteins and appear as signal transducers. Thus, they likely represent novel cell-surface receptors and may play a role in regulating the growth of carcinoma cells.























































 膜杰作
膜杰作 Star Staining
Star Staining
















