State of the Art: Personalizing Treatment for Patients With Metastatic Castration-Resistant Prostate CancerAbida, Beltran, Raychaudhuri
Am Soc Clin Oncol Educ Book (2025) 45 (3), e473636
Abstract: Until recently, the treatment of metastatic castration-resistant prostate cancer (mCRPC) relied exclusively on hormonal therapies and taxane chemotherapy. The advent of modern molecular profiling methods applied in the clinic, namely, next-generation sequencing and advanced positron emission tomography (PET) imaging, has allowed for the development of biomarker-driven therapeutics including anti-PD-L1 therapy for microsatellite instability-high or tumor mutation burden-high disease, poly(ADP-ribose) polymerase (PARP) inhibitors for patients with DNA damage repair mutations, and lutetium 177 vipivotide tetraxetan (177Lu-PSMA-617) for patients with prostate-specific membrane antigen (PSMA) PET-avid disease. While these targeted therapies have improved outcomes, there is an opportunity to refine biomarkers to optimize patient selection, understand resistance, and develop novel combination strategies. In addition, studies in the laboratory and in patient-derived samples have shown that a subset of mCRPC tumors lose expression of common prostate cancer markers such as prostate-specific antigen and PSMA because of lineage plasticity and the development of non-androgen receptor (AR)-driven disease. Non-AR-driven prostate cancer has been associated with aggressive behavior and poor prognosis, including in some cases histologic transformation to a poorly differentiated neuroendocrine prostate cancer (NEPC). The clinical management of NEPC typically follows the treatment paradigm for small cell lung cancer and increasingly relies on genomic and phenotypic characterization of disease, including loss of tumor suppressors and expression of cell surface markers such as DLL3. Therefore, both genomic subtyping and phenotypic subtyping are important to consider and can guide the clinical management of patients with advanced prostate cancer.
Safety and efficacy outcomes of delta‑like ligand 3 inhibitors for the treatment of solid tumors: A systematic review and single‑arm meta‑analysisSu, Lu, Liu
et alOncol Lett (2025) 29 (5), 228
Abstract: The aim of the present study was to evaluate the clinical curative effects and toxicity of existing delta-like ligand 3 (DLL3) inhibitors in advanced solid tumors with high DLL3 expression. A systematic search across major databases was performed, adhering to the Preferred Reporting Items for Systematic reviews and Meta-Analyses guidelines, and included clinical trials that assessed the efficacy and safety of DLL3 inhibitors in treating solid tumors. To be included, studies had to be randomized controlled trials (RCTs), quasi-RCTs, non-randomized comparative studies, single-arm trials and trials in which DLL3 inhibitors were used in both arms. The results of 21 trials, involving a total of 2,452 patients, which evaluated the efficacy of DLL3 inhibitors in treating solid tumors, were analyzed. The median overall survival was 6.54 months and the median progression-free survival (PFS) was 3.54 months. Combination immunotherapy demonstrated a longer PFS of 4.2 months compared with monotherapy, which had a PFS of 3.36 months. The disease control rate and objective response rate were 57 and 21%, respectively, with notable heterogeneity observed across studies. Adverse events were common, affecting 93% of patients, and included cytokine release syndrome (49%), thrombocytopenia (23%) and peripheral edema (28%), with variations depending on the specific inhibitor used. To conclude, DLL3 inhibitors hold promise for patients with elevated DLL3 expression in solid tumors; however, their efficacy and safety exhibit considerable variability, necessitating large-scale, phase III clinical trials to validate and refine therapeutic approaches. The present study was registered with PROSPERO (registration no. CRD42024561815).Copyright: © 2025 Su et al.
Comparative profiling of surgically resected primary tumors and their lymph node metastases in small-cell lung cancerCsende, Ferencz, Boettiger
et alESMO Open (2025) 10 (4), 104514
Abstract: Profiling studies in small-cell lung cancer (SCLC) have mainly focused on primary tumors, omitting the potential molecular changes that might occur during lymphatic metastasis formation. Here, we assessed the molecular discordance between primary SCLCs and corresponding lymph node (LN) metastases in the light of subtype distribution and expression of clinically relevant proteins.Comparative profiling of 32 surgically resected primary SCLCs and their LN metastases was achieved by RNA expression analysis and immunohistochemistry (IHC). In addition to subtype markers (ASCL1, NEUROD1, POU2F3, and YAP1), the expression of nine cancer-specific proteins was evaluated.The selected clinically relevant molecules showed no significant differences in their RNA expression profile when assessing the primary tumors and their corresponding LN metastases. Nevertheless, IHC analyses revealed significantly higher DLL3 expression in the primary tumors than in the LN metastases (P = 0.008). In contrast, NEUROD1 expression was significantly lower in the primary tumors (versus LN metastases, P < 0.001). No statistically significant difference was found by IHC analysis in the case of other clinically relevant proteins. Concerning SCLC molecular subtypes, a change in subtype distribution was detected in 21 cases. Phenotype switching from neuroendocrine (NE) subtypes toward non-NE lesions and from non-NE landscape toward NE subtypes were both detected.Although the molecular landscape of SCLC LN metastases largely resembles that of the tumor of origin, key differences exist in terms of DLL3 and NEUROD1 expression, and in subtype distribution. These diagnostic pitfalls should be considered when establishing the tumors' molecular profile for future clinical trials solely based on LN biopsies.Copyright © 2025 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Current and future perspectives in extensive-stage small-cell lung cancerLim, Ryu, Park
et alTher Adv Med Oncol (2025) 17, 17588359251326705
Abstract: Small-cell lung cancer (SCLC) is a highly aggressive and rapidly proliferative malignancy that has historically had limited therapeutic advancements. Recent advancements in the understanding of SCLC have led to attempts at subtyping the disease based on transcription factor characteristics, offering new insights into its biology and potential therapeutic targets. In addition, significant progress has been made in developing treatment regimens, providing new hope for improved patient outcomes. The introduction of immune checkpoint inhibitors, such as atezolizumab and durvalumab, in combination with traditional chemotherapy, has marked a significant advancement, demonstrating improved overall survival and progression-free survival compared to chemotherapy alone. Despite these advancements, the prognosis for extensive-stage SCLC (ES-SCLC), the more advanced form of SCLC, remains poor, highlighting the critical need for ongoing research and the development of novel therapeutic strategies. New treatment modalities, such as lurbinectedin and anti-Delta-like Canonical Notch Ligand 3 antibodies, are now included in the treatment options for refractory SCLC, and many more treatment strategies involving combination therapies are being studied. Advances in molecular profiling and the identification of biomarkers are aiding in the development of personalized treatment approaches. This review focuses on these recent advancements and emerging strategies in the treatment of ES-SCLC.© The Author(s), 2025.

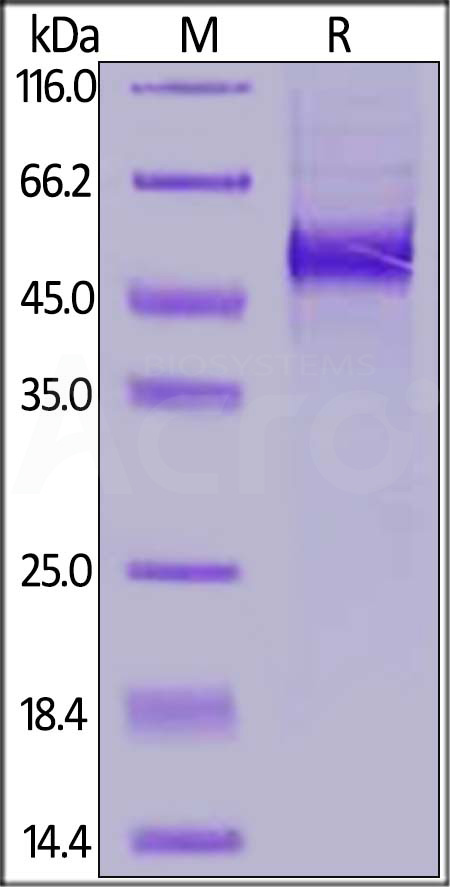

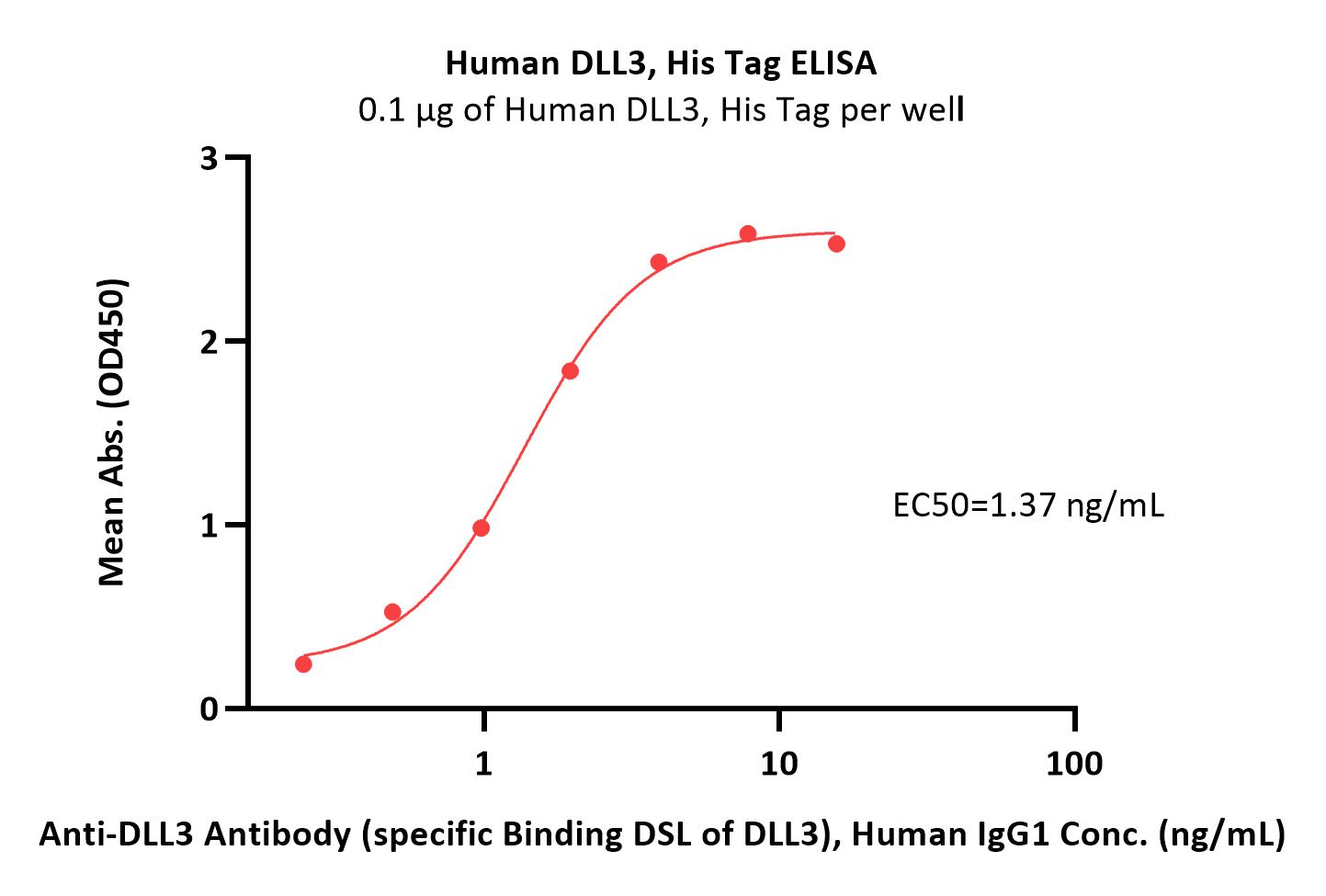



 +添加评论
+添加评论
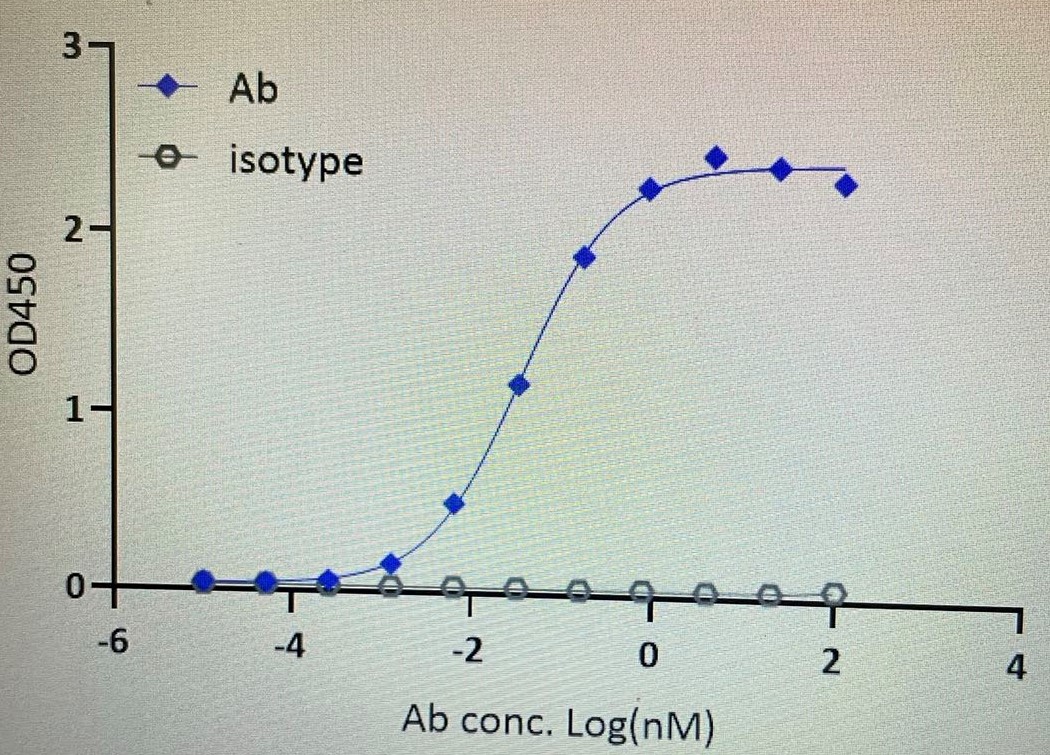 >
>
























































 膜杰作
膜杰作 Star Staining
Star Staining

















