Culturomics of the pig tonsil microbiome identifies new species and an untapped source of novel antimicrobialsde Oliveira, Fredriksen, Gutiérrez
et alMicrobiome (2025) 13 (1), 86
Abstract: In humans and pigs, altered composition of the microbiota associated with the epithelium of the palatine tonsils has been associated with bacterial or viral infection and lymphoid tissue inflammation. Tonsil lymphoid tissue is important for immunity and considered an important portal of entry for pathogens such as Streptococcus suis. Little is known about correlations between tonsil-associated microbiota, tonsillar infections, and the species that might confer colonization resistance against pathogens. Here, we describe a large collection of representative bacterial species from the tonsil surface biofilm and used genome mining and in vitro assays to assess their potential as probiotics to reduce infections by S. suis and other pathogens.Data on tonsil microbiota composition from over 100 piglets from 11 farms and 3 countries revealed a core microbiota comprising Actinobacillus, Streptococcus, and Moraxella and 11 other less abundant but prevalent genera. To establish a collection of culturable core species, we plated 5 tonsil swabs taken from healthy piglets on different farms and countries on 8 different media and isolated 518 pure cultures belonging to 23 genera. To identify candidate probiotic strains, we tested for antagonistic activity against a panel of pathogens and in silico genome mining to find biosynthetic gene clusters (BGCs) in isolates that might produce antimicrobial compounds. We identified two novel species with potential probiotic activities: a Brevibacterium species and Corynebacterium species producing a heat and proteolytically stable lanthipeptide variant of flavucin, inhibiting in vitro growth of the opportunistic pathogens S. suis and Staphylococcus aureus.We defined the core tonsil microbiota of piglets and cultured representative single bacterial isolates for research on microbiota-host interactions in the oral cavity. Several isolates inhibiting the growth of bacterial pathogens that might be exploited as probiotics to promote colonization resistance were deposited in publicly available strain repositories. Our mining of genomes from cultured isolates suggests that the tonsil microbiota is an untapped source of novel antimicrobials Video Abstract.© 2025. The Author(s).
Does facial sunscreen usage impact radiographic image quality and radiation dose? An in vitro studyNejaim, Bregolin, Suekane
et alOral Radiol (2025)
Abstract: To assess whether the use of different types of facial sunscreen influences the quality of radiographic images and the absorbed radiation dose during radiographic acquisitions.In this in vitro study, two types of facial sunscreens (Bioderma®), both with a sun protection factor of 50, were tested: one organic and one inorganic. A polystyrene plate was used, containing a thermoluminescent dosimeter and a photostimulable phosphor plate for the radiographs. The sunscreen was applied to the plate, and five radiographs were taken for each group: control (without sunscreen), organic sunscreen, and inorganic sunscreen. Image quality was assessed by noise, brightness, and uniformity, and the radiation dose was measured in milligrays. The results were compared using one-way analysis of variance with Tukey post-hoc test (α = 5%).Inorganic sunscreen produced images with higher brightness and lower uniformity, with no significant differences in noise. Additionally, this group showed a lower radiation dose (0.50 mGy) compared to the control group (0.60 mGy) and the organic sunscreen (0.58 mGy) (p < 0.05).The inorganic sunscreen altered image quality by increasing brightness and decreasing uniformity, while also reducing the absorbed radiation dose.© 2025. The Author(s) under exclusive licence to Japanese Society for Oral and Maxillofacial Radiology.
Understanding Re-operation in orthognathic surgery: A 17-year retrospective study analyzing causes and ratesSoylu, Kılavuz, Eren
et alJ Craniomaxillofac Surg (2025)
Abstract: Orthognathic surgery is a standard procedure for correcting dentofacial deformities. While it generally yields satisfactory results, complications and re-operations can occur. The purpose of this study was to retrospectively analyze the reasons for the re-operation of patients who previously underwent orthognathic surgery and evaluate the procedures in the second and third surgeries. The patients who underwent orthognathic surgery at Erciyes University between June 2006 and September 2023 were included. The primary outcomes were the reasons for re-operation, and the secondary outcomes were the reasons for plate removal and the location of the removed plates. Reasons for re-operation are classified as plate removal, early malocclusion, late malocclusion, inadequate aesthetic satisfaction, septal deviation, bleeding, and retained foreign bodies. The re-operation rate was found to be 8.33 %. The most common reason for re-operation was plate problems, with 43.53 %. Aesthetic dissatisfaction ranked second among the reasons for re-operation, with 16.47 %. The most common reason for plate removal was infection, with 78.68 %. In conclusion, understanding the underlying causes that may lead to re-operation is crucial in informing patients and minimizing the need for re-operation.Copyright © 2025 European Association for Cranio-Maxillo-Facial Surgery. Published by Elsevier Ltd. All rights reserved.
Precision through Electric-Field Assisted Automatable High Throughput Sample Preparation of Dried Blood Spots for Neonatal Abstinence Syndrome DetectionFariha, Murphy, Walters
et alSLAS Technol (2025)
Abstract: In the United States, approximately 20% of pregnant women disclose opioid misuse, contributing significantly to the widespread occurrence of Neonatal Abstinence Syndrome (NAS) in neonates exposed to opioids during gestation. Current NAS diagnosis heavily relies on clinical observation of symptoms, with the Finnegan Neonatal Abstinence Scoring System (FNASS) serving as the gold standard due to challenges associated with obtaining biological specimens from newborns. This methodological constraint poses difficulties in achieving accurate quantitative assessments and implementing timely therapeutic interventions. This study introduces a pioneering approach employing a cylindrical electrode-equipped device designed for the extraction of opioids from minute Dried Blood Spot (DBS) samples, thus optimizing the diagnostic pathway for NAS. The methodology integrates Liquid Chromatography tandem Mass Spectrometry (LC-MS/MS) for precise quantification of five distinct opioids. By demonstrating the efficacy of DBS microsamples as a robust quantitative diagnostic medium, this research highlights its potential to expedite NAS detection in infants. The innovative methodology promises superior diagnostic precision and accelerated processing times compared to current protocols, thereby addressing existing NAS diagnostic limitations and advancing maternal and infant healthcare practices.Copyright © 2025. Published by Elsevier Inc.

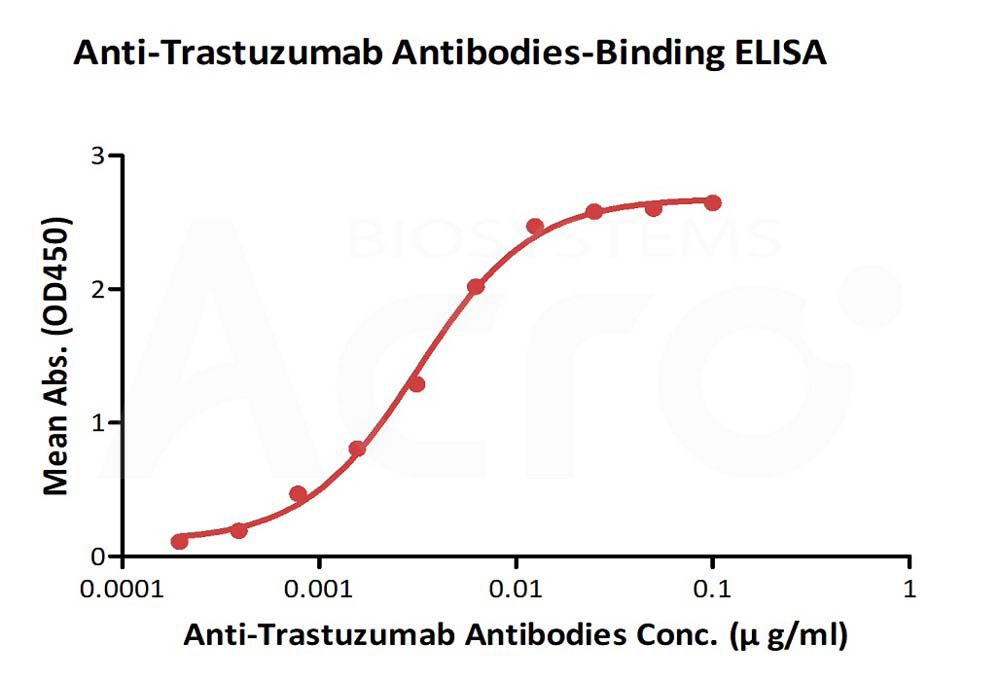
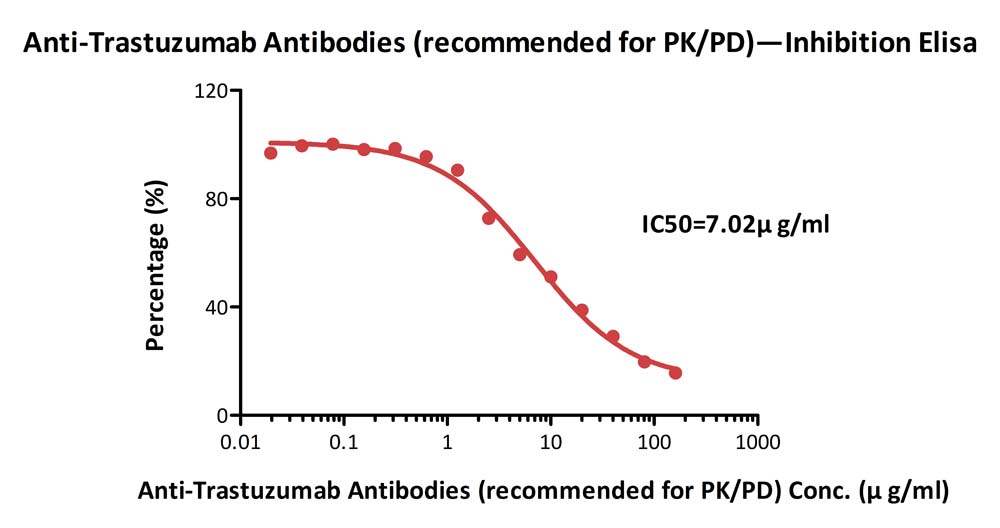
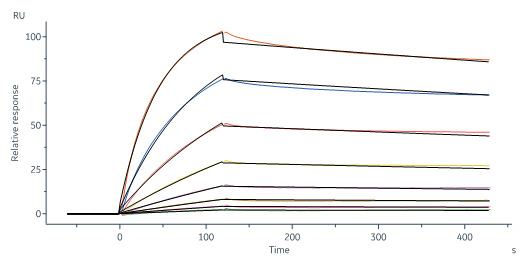
 +添加评论
+添加评论






















































 膜杰作
膜杰作 Star Staining
Star Staining














