分子别名(Synonym)
FGF2,BFGF,FGFB,FGF basic,HBGF-2
表达区间及表达系统(Source)
Human FGF basic, premium grade (BFF-H4117) is expressed from E. coli cells. It contains AA Pro 143 - Ser 288 (Accession # P09038-4).
Predicted N-terminus: Pro 143
It is produced under our rigorous quality control system that incorporates a comprehensive set of tests including sterility and endotoxin tests. Product performance is carefully validated and tested for compatibility for cell culture use or any other applications in the early preclinical stage.
GMP-FGCH17 is the GMP version of this BFF-H4117. These two proteins display indistinguishable performance profiles, thereby ensuring a seamless transition for end users from early preclinical stag to later clinical phases.
Request for sequence
蛋白结构(Molecular Characterization)

This protein carries no "tag".
The protein has a calculated MW of 16.5 kDa. The protein migrates as 17 kDa±3 kDa when calibrated against Star Ribbon Pre-stained Protein Marker under reducing (R) condition (SDS-PAGE).
内毒素(Endotoxin)
Less than 0.01 EU per μg by the LAL method.
宿主蛋白残留(Host Cell Protein)
<0.5 ng/µg of protein tested by ELISA.
宿主核酸残留(Host Cell DNA)
<0.02 ng/μg of protein tested by qPCR.
无菌(Sterility)
Negative
支原体(Mycoplasma)
Negative.
纯度(Purity)
>95% as determined by SDS-PAGE.
>95% as determined by SEC-HPLC.
制剂(Formulation)
Lyophilized from 0.22 μm filtered solution in PBS, pH7.4 with trehalose as protectant.
Contact us for customized product form or formulation.
重构方法(Reconstitution)
Please see Certificate of Analysis for specific instructions.
For best performance, we strongly recommend you to follow the reconstitution protocol provided in the CoA.
存储(Storage)
For long term storage, the product should be stored at lyophilized state at -20°C or lower.
Please avoid repeated freeze-thaw cycles.
This product is stable after storage at:
- -20°C to -70°C for 24 months in lyophilized state;
- -70°C for 3 months under sterile conditions after reconstitution.
质量管理控制体系(QMS)
电泳(SDS-PAGE)
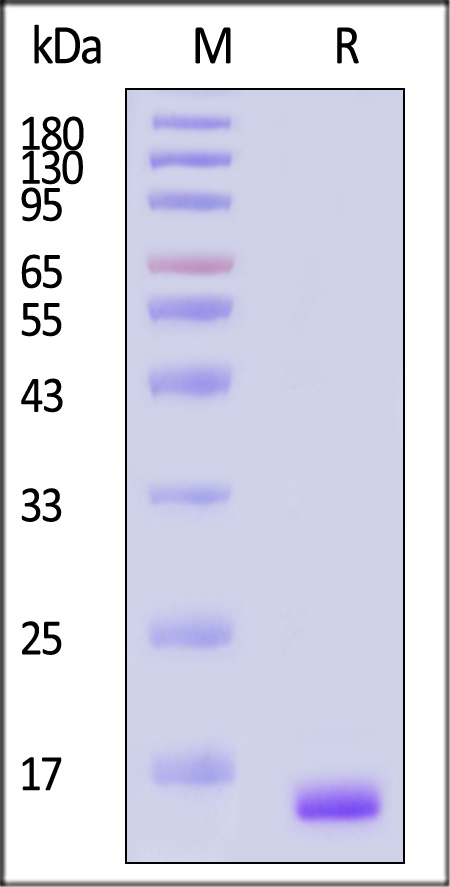
Human FGF basic, premium grade on SDS-PAGE under reducing (R) condition. The gel was stained with Coomassie Blue. The purity of the protein is greater than 95% (With Star Ribbon Pre-stained Protein Marker).
SEC-HPLC
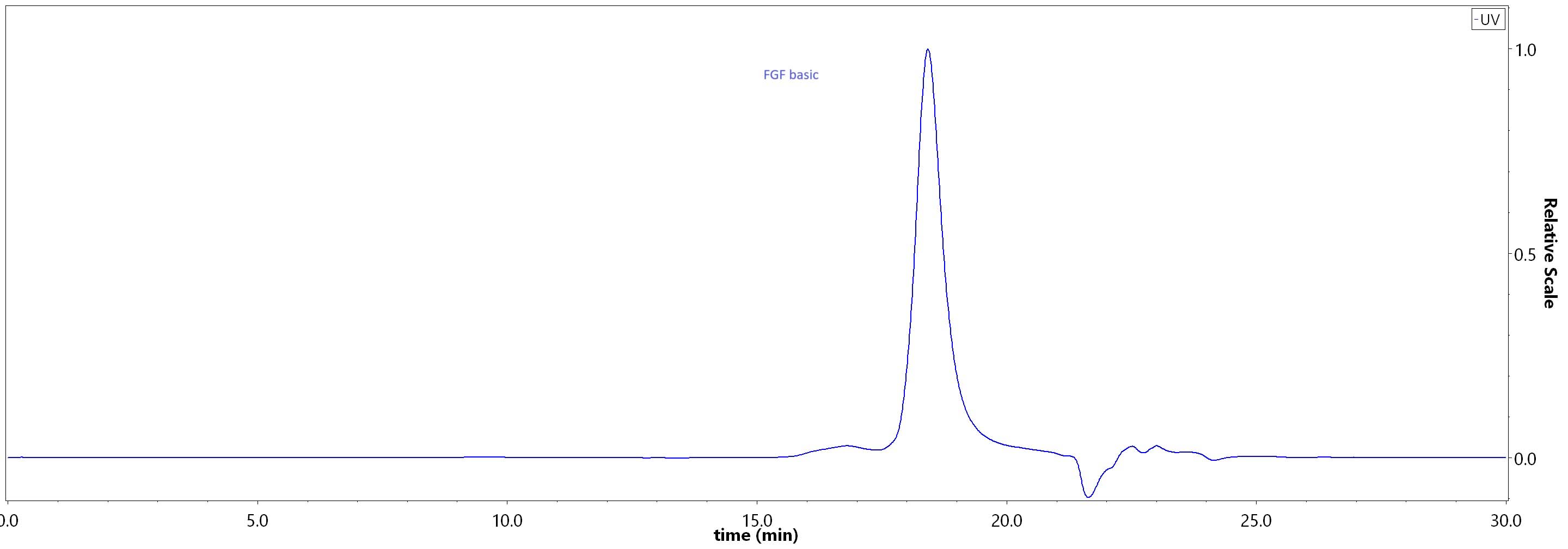
The purity of Human FGF basic, premium grade (Cat. No. BFF-H4117) was greater than 95% as determined by SEC-HPLC.
活性(Bioactivity)-CELL BASE
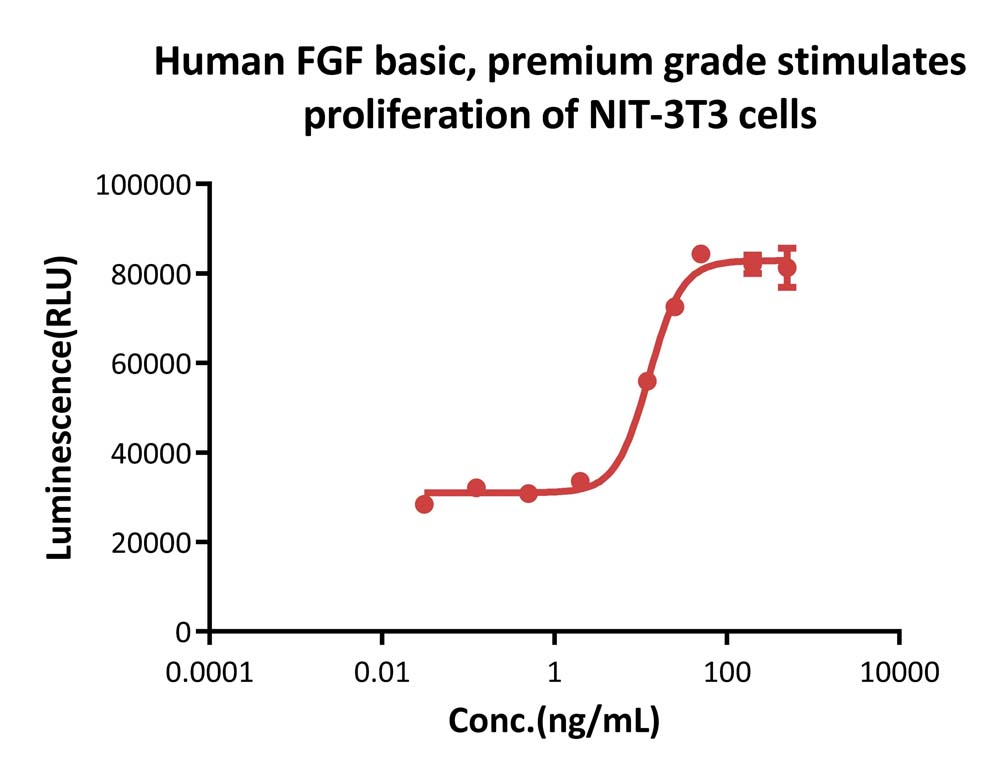
Human FGF basic, premium grade (Cat. No. BFF-H4117) stimulates proliferation of NIH/3T3 cells. The specific activity of Human FGF basic, premium grade is > 2.50×10^6 IU/mg, which is calibrated against human FGF basic WHO International Standard (NIBSC code: 90/712) (QC tested).
Protocol
活性(Bioactivity)-ELISA
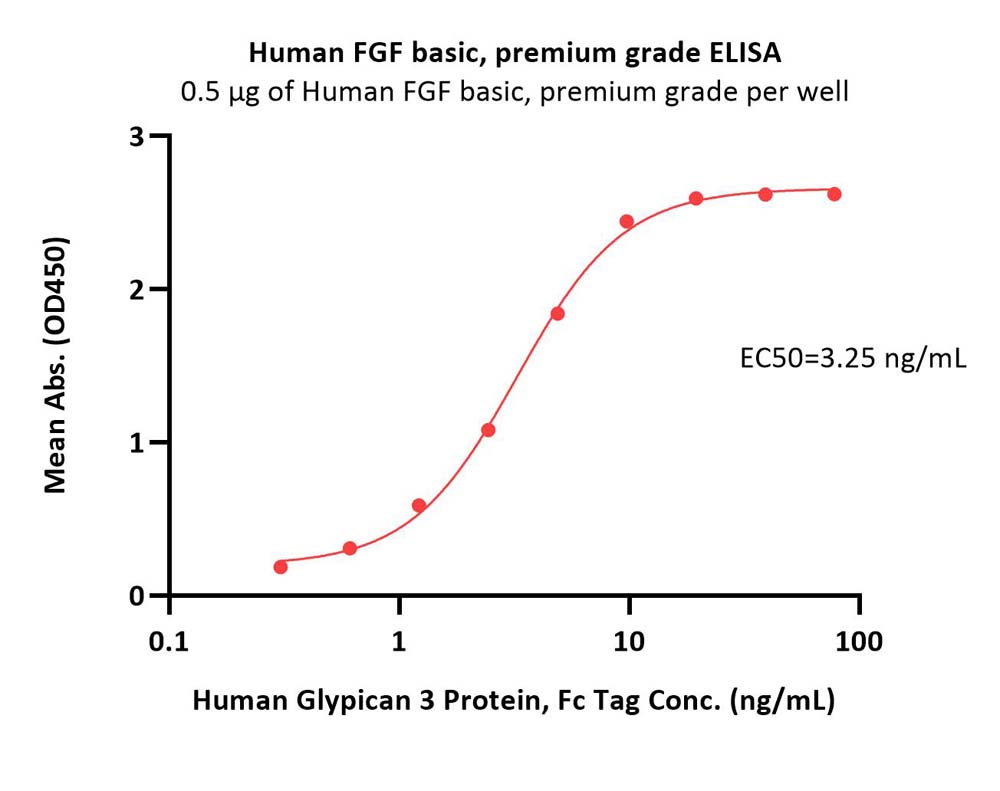
Immobilized Human FGF basic, premium grade (Cat. No. BFF-H4117) at 5 μg/mL (100 μL/well) can bind Human Glypican 3 Protein, Fc Tag (Cat. No. GP3-H5258) with a linear range of 0.3-5 ng/mL (QC tested).
Protocol
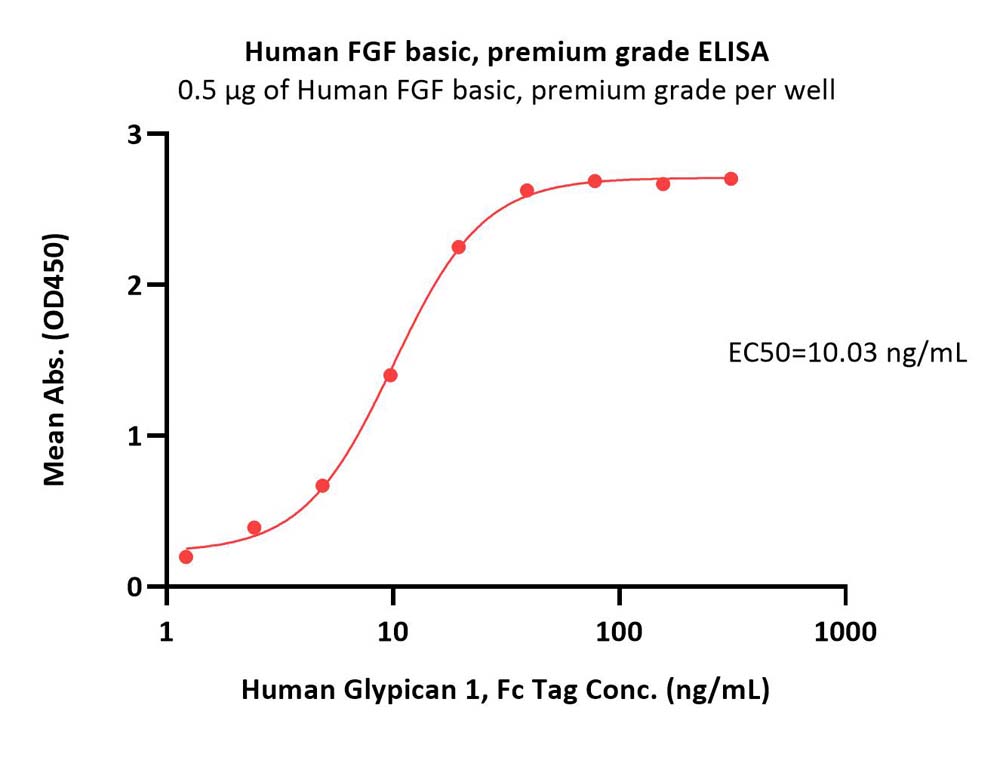
Immobilized Human FGF basic, premium grade (Cat. No. BFF-H4117) at 5 μg/mL (100 μL/well) can bind Human Glypican 1, Fc Tag (Cat. No. GP1-H5254) with a linear range of 1-20 ng/mL (Routinely tested).
Protocol
稳定性(Stability)

The Cell based assay shows that Human FGF basic, premium grade (Cat. No. BFF-H4117) is stable at 37℃ for 48 hours.
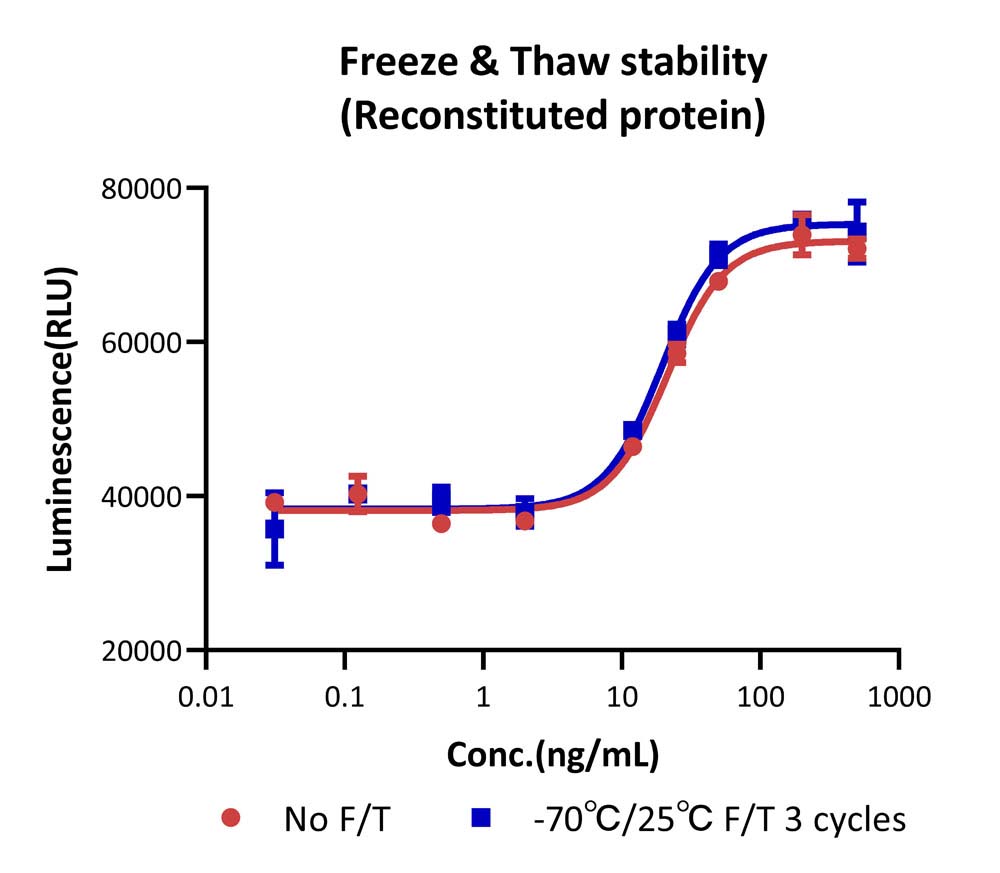
The Cell based assay shows that Human FGF basic, premium grade (Cat. No. BFF-H4117) is stable after freezing and thawing 3 times.

The Cell based assay shows batch-to-batch consistency between Acro's GMP and PG FGF basic.
 +添加评论
+添加评论背景(Background)
FGF basic is a member of the FGF family of at least 23 related mitogenic proteins which show 35-60% amino acid conservation. FGF acidic and basic, unlike the other members of the family, lack signal peptides and are apparently secreted by mechanisms other than the classical protein secretion pathway. FGF basic has been isolated from a number of sources, including neural tissue, pituitary, adrenal cortex, corpus luteum, and placenta. This factor contains four cysteine residues, but reduced FGF basic retains full biological activity, indicating that disulfide bonds are not required for this activity. bFGF is a critical component of human embryonic stem cell culture medium; the growth factor is necessary for the cells to remain in an undifferentiated state, although the mechanisms by which it does this are poorly defined. It has been demonstrated to induce gremlin expression which in turn is known to inhibit the induction of differentiation by bone morphogenetic proteins. It is necessary in mouse-feeder cell dependent culture systems, as well as in feeder and serum-free culture systems.























































 膜杰作
膜杰作 Star Staining
Star Staining















