对于CAR-T细胞来说,发挥肿瘤杀伤作用的有效成分是CAR阳性的T细胞,CAR-T细胞产品的包装规格及临床使用剂量是以CAR-T阳性细胞数表示的,因此,CAR转染阳性率是CAR-T细胞产品质量控制的必检项,监管部门建议应采用流式细胞法检测CAR转染阳性率。目前有针对CAR不同结构区域的检测方法,包括针对CAR抗原结合位点的,比如CD19抗原和抗独特型抗体,或针对轻链或铰链区的抗Fab抗体或Protein L蛋白,其中,针对抗原结合部位的CAR阳性率检测方法因其具有更好专属性而被广泛使用。本文将对CAR阳性表达率检测相关案例做一汇总以供大家参考。
四种CAR-T阳性率检测试剂优劣势对比
|
试剂
|
检测机制
|
优势
|
劣势
|
|
Protein L
|
结合抗体κ轻链
|
具有一定的通用性,可检测不同靶点的CAR
|
仅能识别人VκI VκIII和VκIV亚型和鼠VκI亚型,但不能识别人VκII亚型和鼠Ig的其他亚型轻链
|
|
Anti-Fab抗体
|
结合抗体Fab段
|
具有一定的通用性,可检测不同靶点的CAR
|
背景值高,商品化产品质量参差不齐且多数为多抗,批间一致性较差
|
|
Anti-idiotype抗体
|
特异性结合scFv抗原识别区
|
高特异性、高灵敏度,低背景
|
少有商品化产品,需定制开发,定制周期较长(6个月左右)
|
|
靶点蛋白
|
特异性结合scFv抗原识别区
|
具靶点专属性,可以评估CAR与靶标的结合能力
|
检测灵敏度受scFv与靶点蛋白间的亲和力限制
|
案例汇总
|
案例名称
|
数据来源
|
|
|
案例一
|
利用PE标记CD19蛋白检测anti-CD19 CAR 阳性表达率
|
ACROBiosystems
|
点击查看
|
|
案例二
|
利用PE标记BCMA蛋白检测anti-BCMA CAR 阳性表达率
|
ACROBiosystems
|
点击查看
|
|
案例三
|
利用FITC标记anti-FMC63 antibody检测anti-CD19(FMC63) CAR 阳性表达率
|
ACROBiosystems
|
点击查看
|
|
案例四
|
利用生物素标记hBCMA检测anti-BCMA CAR阳性表达率
|
深圳普瑞金
|
点击查看
|
|
案例五
|
利用生物素标记hCD19检测anti-CD19 CAR阳性表达率
|
MacLeod DT
|
点击查看
|
|
案例六
|
不同供应商hCD19, Fc tag蛋白的结合特异性验证
|
ACROBiosystems
|
点击查看
|
(点击对应案例即可跳转案例详情)
案例详情
案例一、利用PE标记CD19蛋白检测anti-CD19 CAR 阳性表达率
返回案例汇总
检测方法:流式细胞术
检测仪器:BD FACS Celesta流式细胞分析仪 (BD Biosciences)
样本信息:Anti-CD19 CAR-293细胞
主要试剂:
PE标记的CD19蛋白(PE-Labeled Human CD19 (20-291) Protein, His Tag (Site-specific conjugation) ACROBiosystems, Cat. No. CD9-HP2H3);
简要流程:
-
1.用含10% FBS的DMEM培养基在37℃,5% CO2培养箱中培养Anti-CD19 CAR-293细胞。
-
2.收集细胞,进行细胞计数及存活率测定,等分细胞悬液,每管 2×105 活细胞(Anti-CD19-CAR阳性表达率为98%,注意细胞存活率必须大于等于95%)。
-
3.加入100 µL稀释后的PE标记CD19蛋白(ACROBiosystems, Cat. No. CD9-HP2H3)(1:50稀释),4℃避光孵育1小时。
-
4.用含2% BSA的PBS缓冲液洗涤细胞三次,并用200 µL PBS重悬细胞。
-
5.转移细胞至流式管进行流式细胞分析。
检测结果:结果显示Anti-CD19 CAR阳性表达率为100%
FACS Analysis of anti-BCMA CAR expression
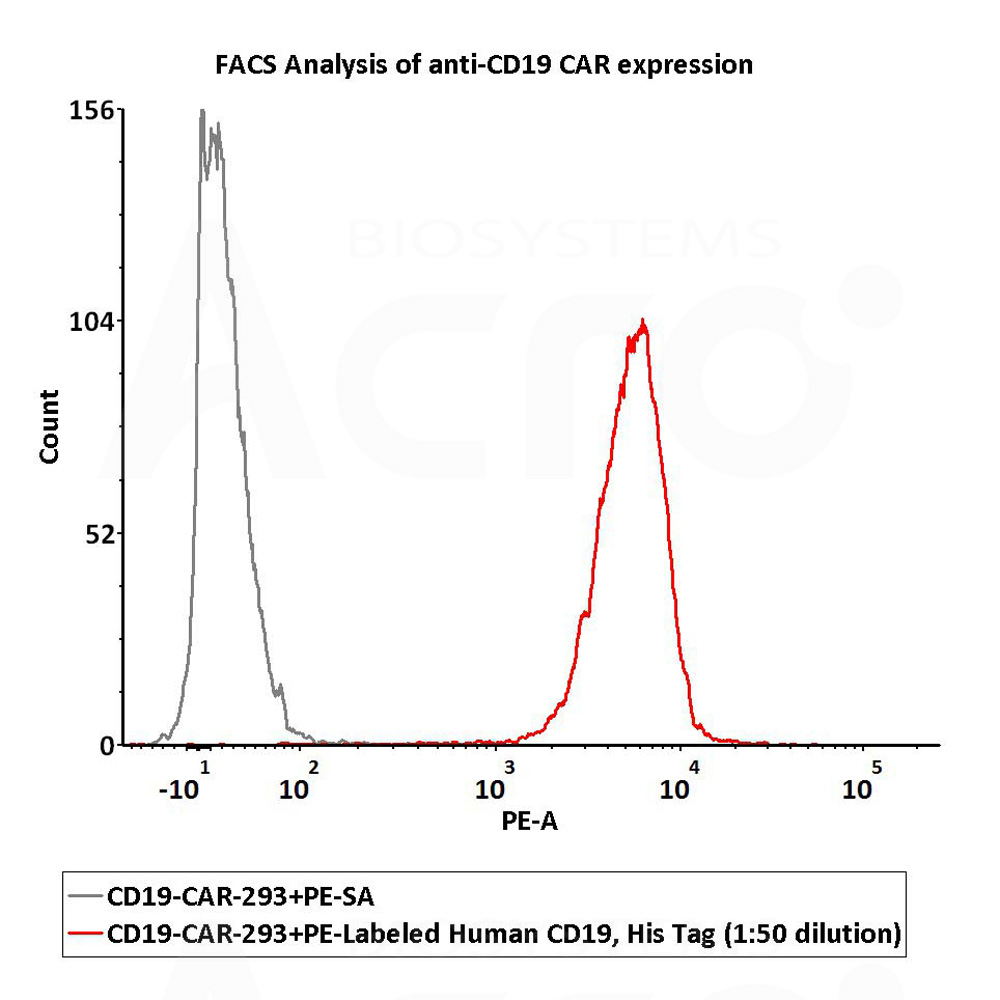 1e6 of the anti-CD19 CAR-293 cells were stained with 100 μL of 1:50 dilution (2 μL stock solution in 100 μL FACS buffer) of PE-Labeled Human CD19 (20-291), His Tag (Cat. No. CD9-HP2H3). PE Streptavidin was used as negative control (QC tested).
1e6 of the anti-CD19 CAR-293 cells were stained with 100 μL of 1:50 dilution (2 μL stock solution in 100 μL FACS buffer) of PE-Labeled Human CD19 (20-291), His Tag (Cat. No. CD9-HP2H3). PE Streptavidin was used as negative control (QC tested).
Protocol
案例二、利用PE标记BCMA蛋白检测anti-BCMA CAR 阳性表达率
返回案例汇总
检测方法:流式细胞术
检测仪器:BD FACS Celesta流式细胞分析仪 (BD Biosciences)
样本信息:Anti-BCMA CAR-293细胞
主要试剂:
PE标记的BCMA蛋白(PE-Labeled Human BCMA / TNFRSF17 Protein, His Tag (Site-specific conjugation) ACROBiosystems, Cat. No. BCA-HP2H2);
简要流程:
-
1.用含10% FBS的DMEM培养基在37℃,5% CO2培养箱中培养Anti-BCMA CAR-293细胞。
-
2.收集细胞,进行细胞计数及存活率测定,等分细胞悬液,每管 2×105 活细胞(Anti-BCMA-CAR阳性表达率为98%,注意细胞存活率必须大于等于95%)。
-
3.加入100 µL稀释后的PE标记BCMA蛋白(ACROBiosystems, Cat. No. BCA-HP2H2)(1:50稀释),4℃避光孵育1小时。
-
4.用含2% BSA的PBS缓冲液洗涤细胞三次,并用200 µL PBS重悬细胞。
-
5.转移细胞至流式管进行流式细胞分析。
检测结果:结果显示Anti-BCMA CAR阳性表达率为100%
FACS Analysis of anti-BCMA CAR expression
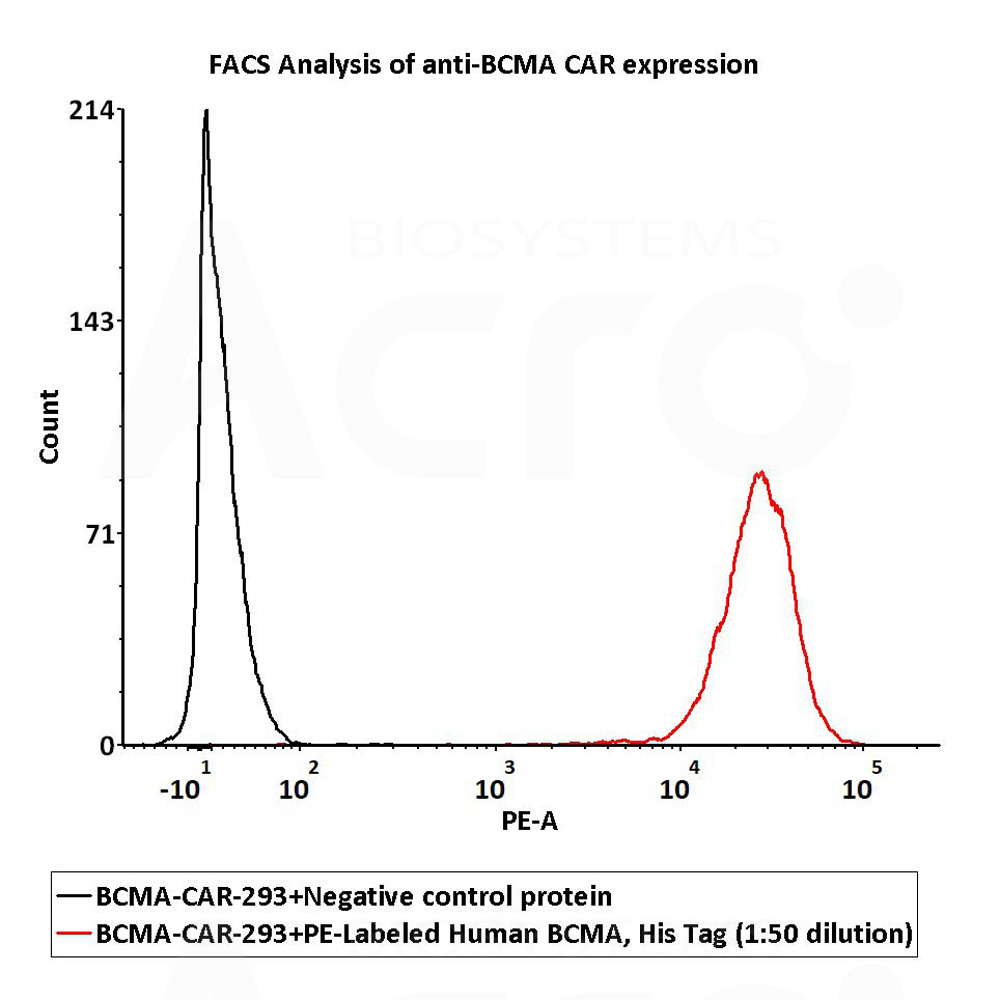 1e6 of the Anti-BCMA CAR-293 cells were stained with 100 μL of 1:50 dilution (2 μL stock solution in 100 μL FACS buffer) of PE-Labeled Human BCMA Protein, His Tag (Cat. No. BCA-HP2H2) and negative control protein respectively, PE signal was used to evaluate the binding activity (QC tested).
1e6 of the Anti-BCMA CAR-293 cells were stained with 100 μL of 1:50 dilution (2 μL stock solution in 100 μL FACS buffer) of PE-Labeled Human BCMA Protein, His Tag (Cat. No. BCA-HP2H2) and negative control protein respectively, PE signal was used to evaluate the binding activity (QC tested).
Protocol
案例三、利用FITC标记anti-FMC63 antibody检测anti-CD19(FMC63) CAR 阳性表达率
返回案例汇总
检测方法:流式细胞术
检测仪器:BD FACS Celesta流式细胞分析仪 (BD Biosciences)
样本信息:Anti-CD19 CAR-293细胞
主要试剂:
FITC标记的抗FMC63抗体(FITC-Labeled Monoclonal Anti-FMC63 scFv Antibody, Mouse IgG1 (Y45) ACROBiosystems, Cat. No. FM3-FY45);
简要流程:
-
1.用含10% FBS的DMEM培养基在37℃,5% CO2培养箱中培养Anti-CD19 CAR-293细胞。
-
2.收集细胞,进行细胞计数及存活率测定,等分细胞悬液,每管 2×105 活细胞(Anti-CD19-CAR阳性表达率为98%,注意细胞存活率必须大于等于95%)。
-
3.加入100 µL稀释后的FITC标记抗FMC63抗体(ACROBiosystems, Cat. No. FM3-FY45)(1:50稀释),4℃避光孵育1小时。
-
4.用含2% BSA的PBS缓冲液洗涤细胞三次,并用200 µL PBS重悬细胞。
-
5.转移细胞至流式管进行流式细胞分析。
检测结果:结果显示Anti-CD19 CAR阳性表达率为100%
FACS Analysis of anti-BCMA CAR expression
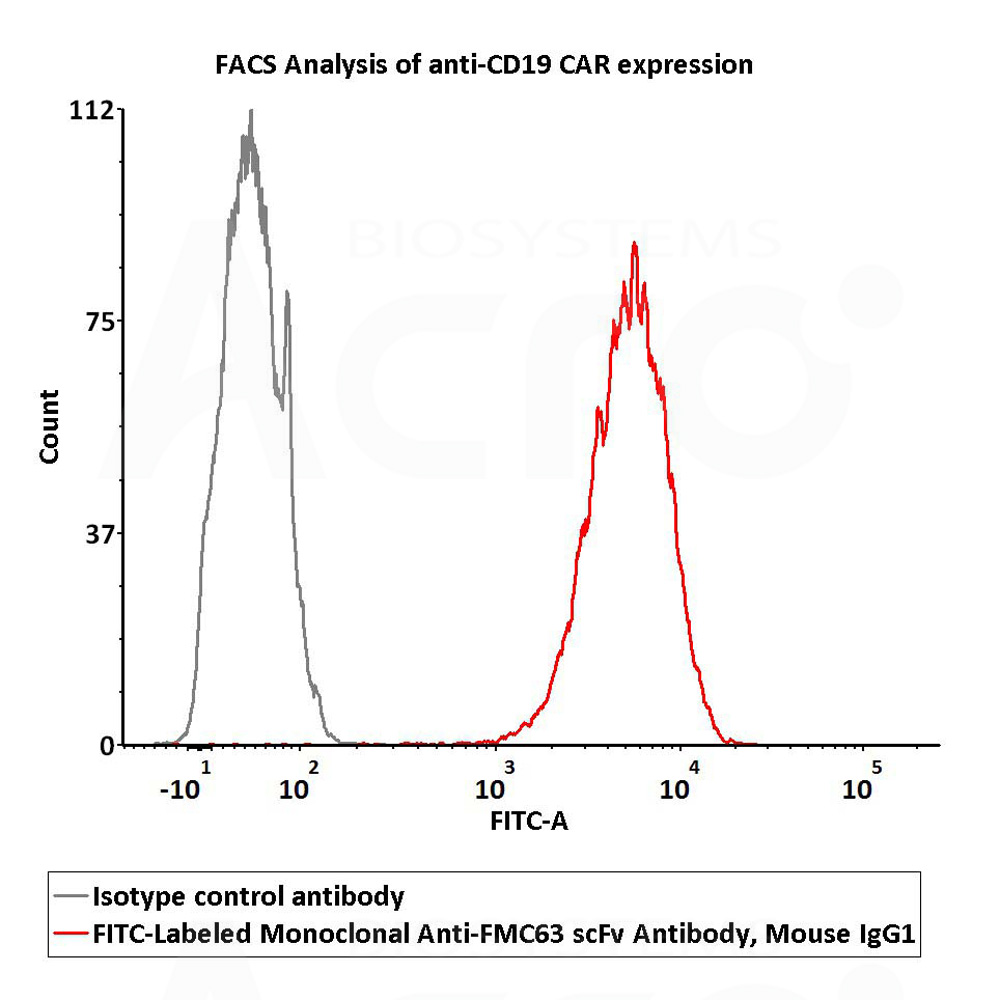 2e5 of Anti-CD19 CAR-293 cells were stained with 100 μL of 1:50 dilution (2 μL stock solution in 100 μL FACS buffer) FITC-Labeled Monoclonal Anti-FMC63 scFv Antibody, Mouse IgG1 (Cat. No. FM3-FY45) and isotype control respectively. FITC signal was used to evaluate the binding activity (QC tested).
2e5 of Anti-CD19 CAR-293 cells were stained with 100 μL of 1:50 dilution (2 μL stock solution in 100 μL FACS buffer) FITC-Labeled Monoclonal Anti-FMC63 scFv Antibody, Mouse IgG1 (Cat. No. FM3-FY45) and isotype control respectively. FITC signal was used to evaluate the binding activity (QC tested).
Protocol
案例四、利用生物素标记hBCMA检测anti-BCMA CAR阳性表达率
返回案例汇总
应用案例由深圳普瑞金生物药业公司友情提供!
检测方法:流式细胞术
检测仪器:BD FACSCalibur流式细胞分析仪
样本信息:转染anti-BCMA CAR的人原代T淋巴细胞
主要试剂:
生物素标记的hBCMA蛋白 (Biotinylated Human BCMA / TNFRSF17 Protein, Fc Tag, Avi Tag (Avitag™), ACROBiosystems, Cat.No. BC7-H82F0);
PE标记的链霉亲和素(PE Streptavidin , Biolegend, Cat.No. 405204);
简要流程:
-
1. 将anti-BCMA CAR基因通过慢病毒质粒转染,整合到来自患者的自体T细胞基因中;
-
2. 利用anti-BCMA CAR特异性结合BCMA蛋白的特性,将生物素标记的hBCMA蛋白(ACROBiosystems, Cat.No. BC7-H82F0)标记到anti-BCMA CAR-T细胞表面;
-
3. 利用生物素特异性结合亲和素的特性,将PE标记的链霉亲和素(Biolegend, Cat.No. 405204) 作为荧光二抗,标记到anti-BCMA CAR-T细胞表面;
-
4. 用BD FACSCalibur流式细胞分析仪进行检测分析。
检测结果:结果显示1号患者anti-BCMA-CAR T细胞阳性率在52.72%,2号患者anti-BCMA-CAR T细胞阳性率在73.49%.
FACS Analysis of anti-BCMA CAR expression
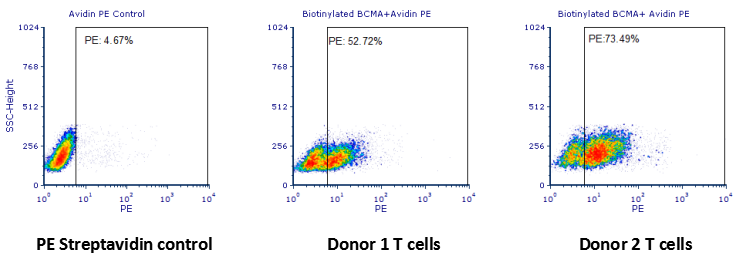 Human T cells were transfected with anti-BCMA CAR and cultured for 3 days. Three days post-transfection, 1x10 6 cells were first incubated with 50ul biotinylated human BCMA protein (Cat.No. BC7-H82F0, 8ug/ml ), washed and
then stained with PE Streptavidin and analyzed by flow cytometry. (Data are kindly provided by PREGENE Biopharma)
Human T cells were transfected with anti-BCMA CAR and cultured for 3 days. Three days post-transfection, 1x10 6 cells were first incubated with 50ul biotinylated human BCMA protein (Cat.No. BC7-H82F0, 8ug/ml ), washed and
then stained with PE Streptavidin and analyzed by flow cytometry. (Data are kindly provided by PREGENE Biopharma)
案例五、利用生物素标记hCD19检测anti-CD19 CAR阳性表达率
返回案例汇总
应用案例来自 MacLeod DT, et al., 2017, Mol Ther. 25(4):949-961.doi: 10.1016/j.ymthe.2017.02.005.
检测方法:流式细胞术
检测仪器:BD Fortessa flow cytometer (BD Biosciences)
样本信息:TRC1-2-treated, AAV:TRAC:CAR-transduced T cells
主要试剂:
生物素标记的hCD19蛋白 (Biotinylated Human CD19, Fc Tag, ultra sensitivity (primary amine labeling), ACROBiosystems, Cat.No. CD9-H8259);
PE标记的链霉亲和素(PE Streptavidin, Biolegend);
Anti-CD3-BV711抗体(Brilliant Violet 711™ anti-human CD3 Antibody, BioLegend).
简要流程:
-
1. 利用CD19 CAR能特异性结合CD19蛋白的特性,如果待检T细胞正常表达了CD19 CAR,生物素标记的CD19-Fc蛋白就能被标记到待检T细胞表面;
-
2. 利用生物素能特异性结合亲和素(PE Streptavidin)和Anti-CD3-BV711抗体能特异性结合CD3分子的特性,如果待检T细胞表面有生物素和CD3分子,链霉亲和素上的PE和Anti-CD3抗体上的BV711荧光标记就能被标记到待检T细胞表面;
-
3. 将制备好的单细胞悬液上机进行流式细胞分析。
检测结果:结果显示CD3阴性T细胞群中CD19 CAR表达的比例要高于CD3阳性T细胞群。
FACS Analysis of anti-CD19 CAR expression
 Activated T cells were electroporated with TRC1-2 mRNA and transduced with AAV:TRAC:CAR at an MOI of 400,000 vg/cell and cultured for 5 days in the presence of IL-2. Five days post-transduction, cells were stained for expression of the CAR using abiotinylated CD19-Fc reagent and CD3, with TRC1-2-treated, mock-transduced cells used as a control for gating of CAR expression. CD3+ cells were then depleted. Enriched CD3 cells were cultured for 3 additional days in the presence of IL-15 and IL-21 and then analyzed again by flow cytometry for CD3 and CAR expression.
Activated T cells were electroporated with TRC1-2 mRNA and transduced with AAV:TRAC:CAR at an MOI of 400,000 vg/cell and cultured for 5 days in the presence of IL-2. Five days post-transduction, cells were stained for expression of the CAR using abiotinylated CD19-Fc reagent and CD3, with TRC1-2-treated, mock-transduced cells used as a control for gating of CAR expression. CD3+ cells were then depleted. Enriched CD3 cells were cultured for 3 additional days in the presence of IL-15 and IL-21 and then analyzed again by flow cytometry for CD3 and CAR expression.
案例六、不同供应商hCD19, Fc tag蛋白的结合特异性验证
返回案例汇总
验证数据由ACRO应用开发团队自主研发提供!
检测方法:流式细胞术
细胞样本: R1013-C6 cells (过表达Anti-CD19[FMC63] scFv & RFP的Expi 293细胞);Expi 293 cells;Jurkat E6.1 cells.
主要试剂:
蛋白样本1:Human CD19 Protein, Fc Tag (ACROBiosystems, Cat.No. CD9-H5259);
蛋白样本2:Human CD19 Protein, Fc Tag (Company N);
阴性对照蛋白:Human PD-L1 Protein, Fc Tag (ACROBiosystems, Cat.No. PD1-H5258);
荧光二抗:FITC anti-human IgG Fc antibody (Biolegend, Cat.No. 409310).
简要流程:
-
1. 用Human CD19 Fc tag蛋白分别标记R1013-C6 细胞、Expi 293细胞和Jurkat E6.1细胞;
-
2. 将FITC anti-human IgG Fc antibody作为荧光二抗,对以上细胞进行二次标记;
-
3. 用NovoCyteTM Flow Cytometer流式细胞分析仪进行检测分析。
检测结果:结果显示ACROBiosystems的Human CD19 Protein, Fc Tag蛋白仅能特异性识别R1013-C6细胞表面的Anti-CD19[FMC63] scFv,不能与Expi 293细胞和Jurkat E6.1细胞发生非特异性结合反应;而同等实验条件下,Company N的Human CD19 Protein, Fc Tag蛋白与Expi 293细胞和Jurkat E6.1细胞发生了较强的非特异性结合。
Protocol
Binding specificity analysis of ACROBiosystems hCD19(C-Fc tag) protein
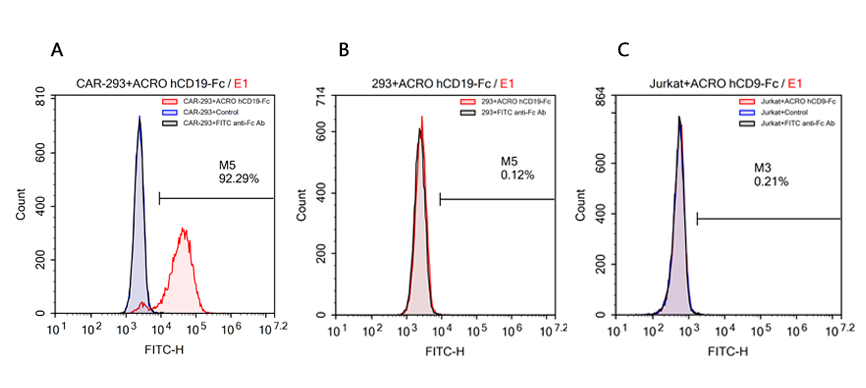 FACS analysis of human CD19 protein, Fc Tag (ACROBiosystems, Cat.No. CD9-H5259) binding to A. R1013-C6 cells, B. Expi 293 cells, C. Jurkat E6.1 cells. Cells were first stained with human CD19 protein, Fc Tag (ACROBiosystems, Cat.No. CD9-H5259) followed by FITC anti-human IgG Fc antibody, and then analyzed using NovoCyteTM Flow Cytometer. The data were analyzed with FCS Express 6Plus and GraphPad Prism 5 software.
FACS analysis of human CD19 protein, Fc Tag (ACROBiosystems, Cat.No. CD9-H5259) binding to A. R1013-C6 cells, B. Expi 293 cells, C. Jurkat E6.1 cells. Cells were first stained with human CD19 protein, Fc Tag (ACROBiosystems, Cat.No. CD9-H5259) followed by FITC anti-human IgG Fc antibody, and then analyzed using NovoCyteTM Flow Cytometer. The data were analyzed with FCS Express 6Plus and GraphPad Prism 5 software.
Protocol
Binding specificity analysis of Company N hCD19(C-Fc tag) protein
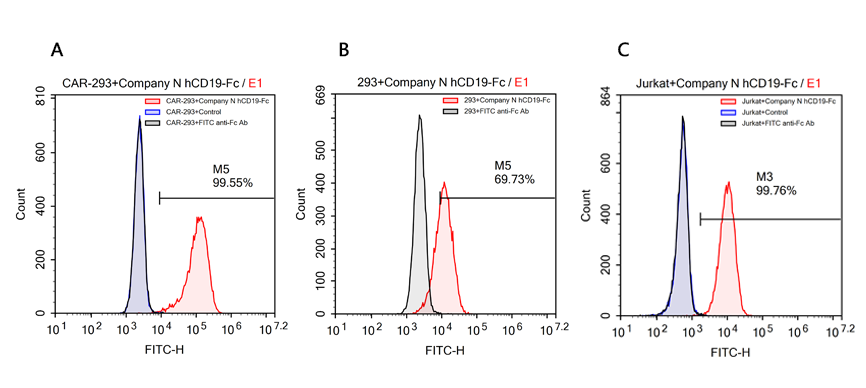 FACS analysis of human CD19 protein, Fc Tag (Company N) binding to A. R1013-C6 cells, B. Expi 293 cells, C. Jurkat E6.1 cells. Cells were first stained with human CD19 protein, Fc Tag (Company N) followed by FITC anti-human IgG Fc antibody, and then analyzed using NovoCyteTM Flow Cytometer. The data were analyzed with FCS Express 6Plus and GraphPad Prism 5 software.
FACS analysis of human CD19 protein, Fc Tag (Company N) binding to A. R1013-C6 cells, B. Expi 293 cells, C. Jurkat E6.1 cells. Cells were first stained with human CD19 protein, Fc Tag (Company N) followed by FITC anti-human IgG Fc antibody, and then analyzed using NovoCyteTM Flow Cytometer. The data were analyzed with FCS Express 6Plus and GraphPad Prism 5 software.






















 Star Ribbon预染蛋白Marker蛋白质标记物是生物研究和药物开发的重要组成部分。无论是用于蛋白质电泳还是western blot,我们的预染色蛋白质标记物帮助您快速确定目标蛋白质的分子量或评估转移效率。Fc受体蛋白治疗性抗体的功效取决于Fab片段及其对目标抗原的结合活性,还取决于Fc片段及其与关键Fc受体的相互作用。因此,在抗体工程中候选物必须针对一系列受体进行测试。探索我们的重组Fc受体蛋白质的全面收藏!
Star Ribbon预染蛋白Marker蛋白质标记物是生物研究和药物开发的重要组成部分。无论是用于蛋白质电泳还是western blot,我们的预染色蛋白质标记物帮助您快速确定目标蛋白质的分子量或评估转移效率。Fc受体蛋白治疗性抗体的功效取决于Fab片段及其对目标抗原的结合活性,还取决于Fc片段及其与关键Fc受体的相互作用。因此,在抗体工程中候选物必须针对一系列受体进行测试。探索我们的重组Fc受体蛋白质的全面收藏!































 膜杰作
膜杰作 Star Staining
Star Staining






 1e6 of the anti-CD19 CAR-293 cells were stained with 100 μL of 1:50 dilution (2 μL stock solution in 100 μL FACS buffer) of PE-Labeled Human CD19 (20-291), His Tag (Cat. No.
1e6 of the anti-CD19 CAR-293 cells were stained with 100 μL of 1:50 dilution (2 μL stock solution in 100 μL FACS buffer) of PE-Labeled Human CD19 (20-291), His Tag (Cat. No.  1e6 of the Anti-BCMA CAR-293 cells were stained with 100 μL of 1:50 dilution (2 μL stock solution in 100 μL FACS buffer) of PE-Labeled Human BCMA Protein, His Tag (Cat. No.
1e6 of the Anti-BCMA CAR-293 cells were stained with 100 μL of 1:50 dilution (2 μL stock solution in 100 μL FACS buffer) of PE-Labeled Human BCMA Protein, His Tag (Cat. No.  2e5 of Anti-CD19 CAR-293 cells were stained with 100 μL of 1:50 dilution (2 μL stock solution in 100 μL FACS buffer) FITC-Labeled Monoclonal Anti-FMC63 scFv Antibody, Mouse IgG1 (Cat. No.
2e5 of Anti-CD19 CAR-293 cells were stained with 100 μL of 1:50 dilution (2 μL stock solution in 100 μL FACS buffer) FITC-Labeled Monoclonal Anti-FMC63 scFv Antibody, Mouse IgG1 (Cat. No.  Human T cells were transfected with anti-BCMA CAR and cultured for 3 days. Three days post-transfection, 1x10 6 cells were first incubated with 50ul biotinylated human BCMA protein (Cat.No.
Human T cells were transfected with anti-BCMA CAR and cultured for 3 days. Three days post-transfection, 1x10 6 cells were first incubated with 50ul biotinylated human BCMA protein (Cat.No.  Activated T cells were electroporated with TRC1-2 mRNA and transduced with AAV:TRAC:CAR at an MOI of 400,000 vg/cell and cultured for 5 days in the presence of IL-2. Five days post-transduction, cells were stained for expression of the CAR using abiotinylated CD19-Fc reagent and CD3, with TRC1-2-treated, mock-transduced cells used as a control for gating of CAR expression. CD3+ cells were then depleted. Enriched CD3 cells were cultured for 3 additional days in the presence of IL-15 and IL-21 and then analyzed again by flow cytometry for CD3 and CAR expression.
Activated T cells were electroporated with TRC1-2 mRNA and transduced with AAV:TRAC:CAR at an MOI of 400,000 vg/cell and cultured for 5 days in the presence of IL-2. Five days post-transduction, cells were stained for expression of the CAR using abiotinylated CD19-Fc reagent and CD3, with TRC1-2-treated, mock-transduced cells used as a control for gating of CAR expression. CD3+ cells were then depleted. Enriched CD3 cells were cultured for 3 additional days in the presence of IL-15 and IL-21 and then analyzed again by flow cytometry for CD3 and CAR expression.
 FACS analysis of human CD19 protein, Fc Tag (ACROBiosystems, Cat.No.
FACS analysis of human CD19 protein, Fc Tag (ACROBiosystems, Cat.No.  FACS analysis of human CD19 protein, Fc Tag (Company N) binding to A. R1013-C6 cells, B. Expi 293 cells, C. Jurkat E6.1 cells. Cells were first stained with human CD19 protein, Fc Tag (Company N) followed by FITC anti-human IgG Fc antibody, and then analyzed using NovoCyteTM Flow Cytometer. The data were analyzed with FCS Express 6Plus and GraphPad Prism 5 software.
FACS analysis of human CD19 protein, Fc Tag (Company N) binding to A. R1013-C6 cells, B. Expi 293 cells, C. Jurkat E6.1 cells. Cells were first stained with human CD19 protein, Fc Tag (Company N) followed by FITC anti-human IgG Fc antibody, and then analyzed using NovoCyteTM Flow Cytometer. The data were analyzed with FCS Express 6Plus and GraphPad Prism 5 software.


