分子别名(Synonym)
OSM,MGC20461,Oncostatin M
表达区间及表达系统(Source)
Human Oncostatin M Protein, premium grade (OSM-H5213) is expressed from human 293 cells (HEK293). It contains AA Ala 26 - Arg 252 (Accession # NP_065391.1).
Predicted N-terminus: Ala 26
It is produced under our rigorous quality control system that incorporates a comprehensive set of tests including sterility and endotoxin tests. Product performance is carefully validated and tested for compatibility for cell culture use or any other applications in the early preclinical stage. When ready to transition into later clinical phases, we also offer a custom GMP protein service that tailors to your needs. We will work with you to customize and develop a GMP-grade product in accordance with your requests that also meets the requirements for raw and ancillary materials use in cell manufacturing of cell-based therapies.
Request for sequence
蛋白结构(Molecular Characterization)

This protein carries no "tag".
The protein has a calculated MW of 25.8 kDa. The protein migrates as 36 kDa under reducing (R) condition (SDS-PAGE) due to glycosylation.
内毒素(Endotoxin)
Less than 0.1 EU per μg by the LAL method.
无菌(Sterility)
Negative
纯度(Purity)
>95% as determined by SDS-PAGE.
制剂(Formulation)
Lyophilized from 0.22 μm filtered solution in PBS, pH7.4 with trehalose as protectant.
Contact us for customized product form or formulation.
重构方法(Reconstitution)
Please see Certificate of Analysis for specific instructions.
For best performance, we strongly recommend you to follow the reconstitution protocol provided in the CoA.
存储(Storage)
For long term storage, the product should be stored at lyophilized state at -20°C or lower.
Please avoid repeated freeze-thaw cycles.
This product is stable after storage at:
- -20°C to -70°C for 12 months in lyophilized state;
- -70°C for 3 months under sterile conditions after reconstitution.
质量管理控制体系(QMS)
电泳(SDS-PAGE)
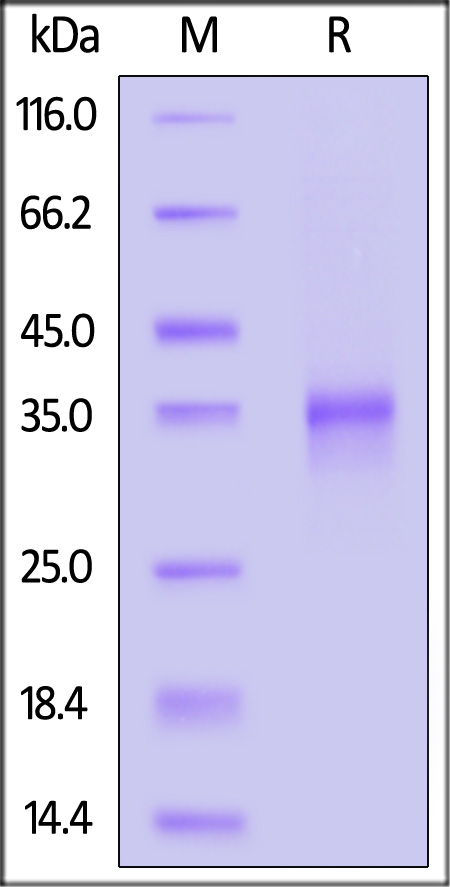
Human Oncostatin M Protein, premium grade on SDS-PAGE under reducing (R) condition. The gel was stained with Coomassie Blue. The purity of the protein is greater than 95%.
活性(Bioactivity)-ELISA
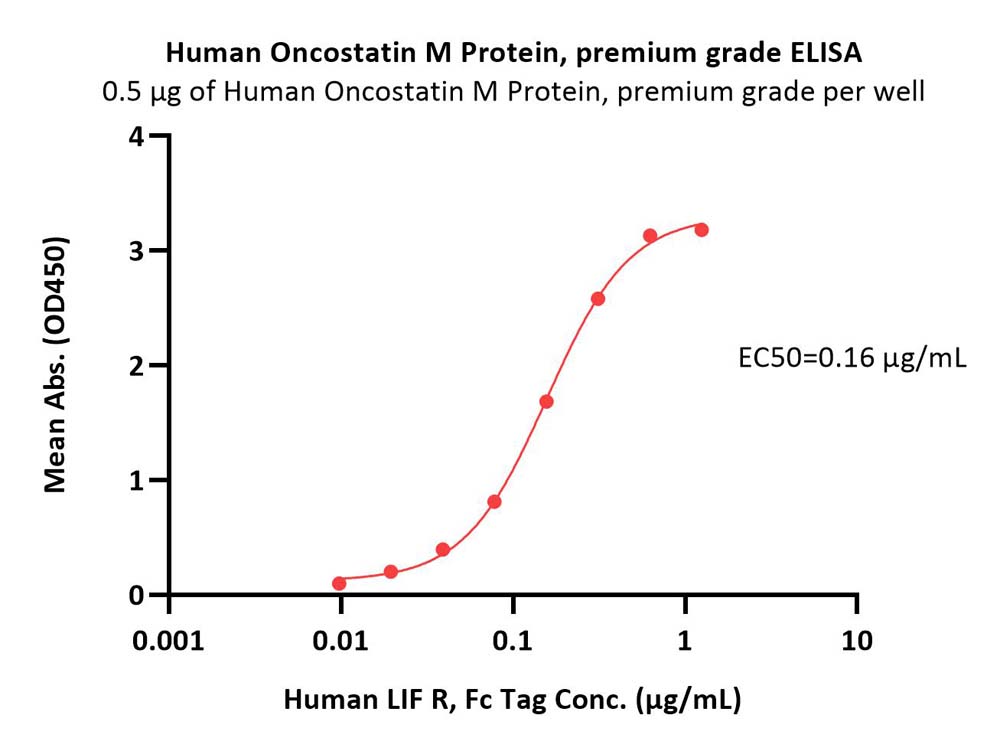
Immobilized Human Oncostatin M Protein, premium grade (Cat. No. OSM-H5213) at 5 μg/mL (100 μL/well) can bind Human LIF R, Fc Tag (Cat. No. LIR-H4252) with a linear range of 0.01-0.312 μg/mL (QC tested).
Protocol
背景(Background)
Oncostatin M is also known as OSM, is a glycoprotein belonging to the interleukin-6 family of cytokines that has functions mainly in cell growth. Of these cytokines it most closely resembles leukemia inhibitory factor (LIF) in both structure and function. However, it is as yet poorly defined and is proving important in liver development, haematopoeisis, inflammation and possibly CNS development. It is also associated with bone formation and destruction. OSM signals through cell surface receptors that contain the protein gp130. The type I receptor is composed of gp130 and LIFR, the type II receptor is composed of gp130 and OSMR. Oncostatin M (OSM) was previoustly identified by its ability to inhibit the growth of cells from melanoma and other solid tumors. It also has been reported that OSM, like LIF, IL-6 and G-CSF, has the ability to inhibit the proliferation of murine M1 myeloid leukemic cells and can induce their differentiation into macrophage-like cells. The human form of OSM is insensitive between pH2 and 11 and resistant to heating for one hour at 56 degree but is not stable at 90 degrees. The three dimensional structure of human OSM has been solved to atomic resolution, confirming the predicted long chain four helix bundle topology. Comparing this structure with the known structures of other known LC cytokines shows it to be most closely related to LIF.























































 膜杰作
膜杰作 Star Staining
Star Staining
















