Revealing the effects of various immune cells in anorexia nervosa: Evidence from Mendelian randomizationDeng, Yu, Yin
et alMedicine (Baltimore) (2025) 104 (12), e41817
Abstract: The aim of this study was to assess the causal relationship between immune cells and anorexia nervosa (AN) by Mendelian randomization (MR). Data on immune cell phenotypes and AN were obtained from genome-wide association studies. Next, single nucleotide polymorphisms included in the MR analysis were screened according the basic assumptions. Furthermore, inverse variance weighted was used as the main method for MR analysis to evaluate the causal effect of immune cell phenotypes on AN. Finally, MR-Egger intercept, Cochran Q, and leave-one-out sensitivity analyses were used to assess horizontal pleiotropy, heterogeneity, and robustness, respectively. The MR analysis showed that NKT %lymphocyte (OR 1.201, 95% CI = 1.021-1.411, P = .027), NKT %T cell (OR 1.258, 95%CI 1.043-1.519, P = .017), double negative (DN) (CD4-CD8-) NKT %lymphocyte (OR 1.235, 95%CI 1.074-1.420, P = .003), DN (CD4-CD8-) NKT %T cell (OR 1.222, 95%CI 1.060-1.410, P = .006), CD8dim NKT absolute count (OR 1.225, 95%CI 1.045-1.436, P = .012), CD8dim NKT %lymphocyte (OR 1.214, 95%CI 1.043-1.414, P = .012), CD8dim NKT %T cell (OR 1.215, 95%CI 1.035-1.425, P = .017), CD16-CD56 on NKT (OR 1.193, 95%CI 1.014-1.402, P = .033), CD28 + CD45RA + CD8br %T cell (OR 1.020, 95%CI 1.002-1.037, P = .025) were associated with increased genetic susceptibility to AN. MR-Egger showed no horizontal pleiotropy (P ≥ .05). Cochran Q and sensitivity analysis showed that the results were not heterogeneous and were robust. This MR analysis revealed 9 immune cell phenotypes related to increased genetic susceptibility to AN, emphasizing the importance of NKT and CD8 in AN. This finding provides new insights for understanding the pathogenesis of AN and developing immune-targeted drugs.Copyright © 2025 the Author(s). Published by Wolters Kluwer Health, Inc.
Genetic and Epigenetic Changes in Melanoma Progression: A TCGA-based StudyCakir, Lebe, Toper
et alAppl Immunohistochem Mol Morphol (2025)
Abstract: We aimed to investigate molecular mechanisms affecting melanoma progression by comparing genetic/epigenetic features between melanomas of different Breslow thickness and stage using TCGA (The Cancer Genome Atlas) data. The TCGA, Firehose Legacy, melanoma data set was utilized on the cBioPortal website. The cases were compared in terms of mRNA expression and DNA methylation. Gene Ontology (GO) and KEGG pathways enrichment analysis were performed using the online WebGestalt tool. STRING and Cytoscape software were used to construct a protein-protein interaction network and identify hub genes. P and q<0.05, FDR< 0.05 were considered statistically significant. 1001 differentially expressed genes were identified between thin (≤1 mm) and thick (>1 mm) melanomas. Pathway analyses revealed that genes enriched in thin melanomas were associated with adaptive immune response, T-cell activation, immune response regulation, leukocyte, and cytokine-related pathways, whereas genes enriched in thick melanomas were related to epidermis development. Ten hub genes were identified (CD4, IFNG, PTPRC, CD8A, CTLA4, CD69, ICOS, CD27, CD28, CD19). All of these genes are involved in crucial immunological processes. Understanding the complex changes in melanoma progression is essential for accurate diagnosis and prediction of prognosis. Our results may shed light on subsequent studies to identify the steps in melanoma progression.Copyright © 2025 Wolters Kluwer Health, Inc. All rights reserved.
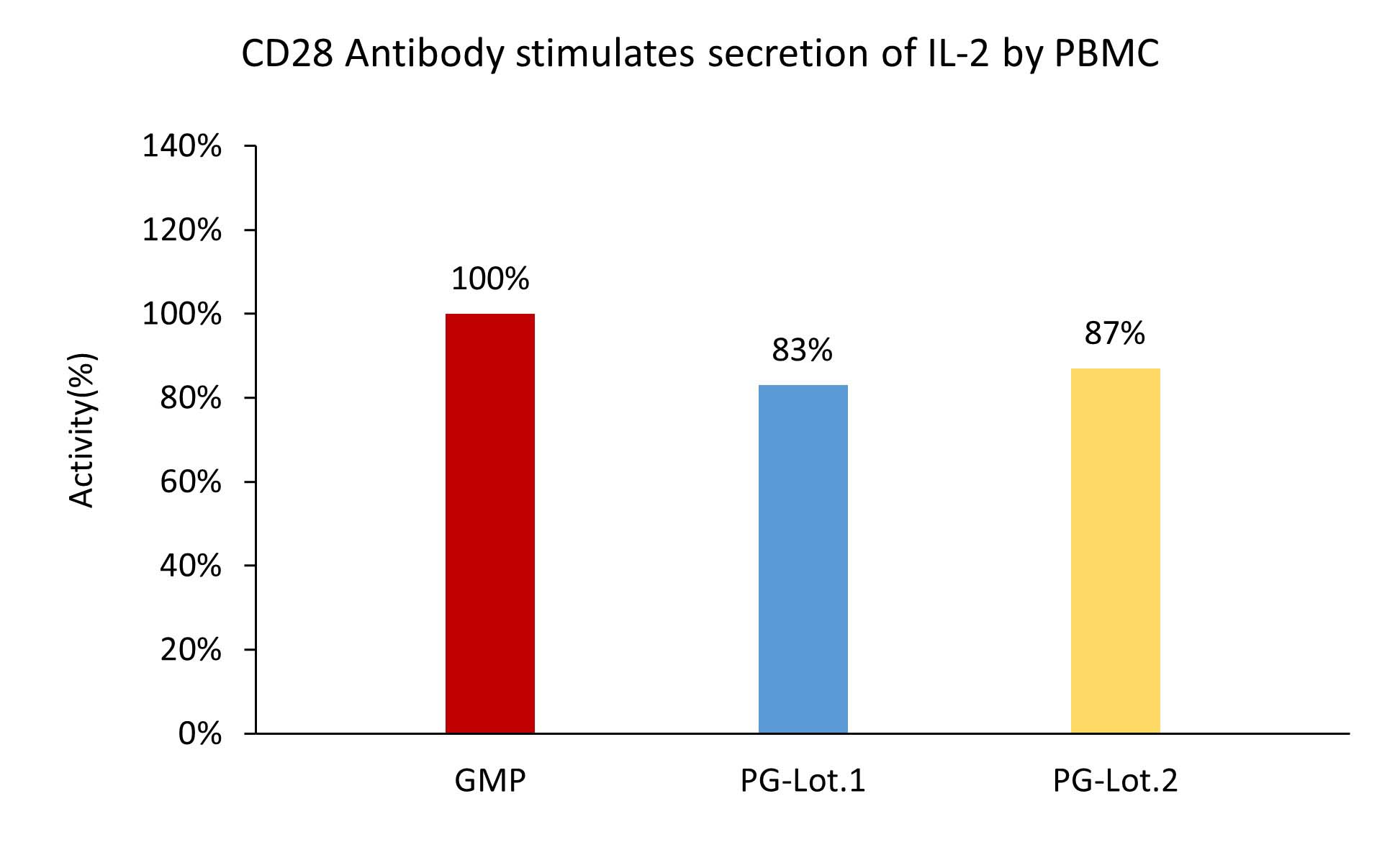
Analysis of Human T Cell Activity in an Allogeneic Co-Culture Setting of Pre-Treated Tumor CellsDonaubauer, Schäfer, Hundsdorfer
et alJ Vis Exp (2025)
Abstract: Cytotoxic T cells play a key role in the elimination of tumor cells and are, therefore, intensively studied in cancer immunology. The frequency and activity of cytotoxic T cells within tumors and their tumor microenvironment (TME) are now well-established prognostic and predictive biomarkers for numerous tumor types. However, it is well-known that various tumor treatment modalities, including radiotherapy, chemotherapy, immunotherapy, and targeted therapy, modulate not only the immunogenicity of the tumor but also the immune system itself. Consequently, the interaction between tumor cells and T cells requires more intensive study in different therapeutic contexts to fully understand the complex role of T cells during tumor therapy. To address this need, a protocol was developed to analyze the activity and proliferative capacity of human cytotoxic (CD8+) T cells in co-culture with pre-treated tumor cells. Specifically, CD8+ T cells from healthy donors are stained with the non-toxic proliferation marker carboxyfluorescein diacetate succinimidyl ester (CFSE) and stimulated using CD3/CD28-coated plates. Subsequently, T cells are co-cultured with pre-treated tumor cells. As a readout, T cell proliferation is quantified by measuring CFSE signal distribution and assessing the expression of surface activation markers via flow cytometry. This can be further complemented by quantifying cytokine release using enzyme-linked immunosorbent assay (ELISA). This method facilitates the evaluation of treatment-induced changes in the interaction between tumor cells and T cells, providing a foundation for more detailed analyses of tumor treatment modalities and their immunogenicity in a human ex vivo setting. Additionally, it contributes to the reduction of pre-clinical in vivo analyses.
Unveiling Potential Blood Markers for Endometriosis Through the Integration and Experimental Validation of Immune Cell Traits Genome and Genome-Wide Associated DataMei, Jiang, Zhang
et alInt J Womens Health (2025) 17, 845-853
Abstract: While endometriosis (EM) has been previously associated with multiple immune factors, the causal relationship underlying these associations remains unclear.In this study, Two-sample Mendelian randomization (MR) method was employed to investigate the causal relationship between 731 immune cell traits and EM based on tabulated data from genome-wide association studies (GWAS).MR method includes inverse variance weighting (IVW), the weighted median (WM), MR-Egger, the weighted model, and the simple model. IVW is used as the primary method for judging causal effects. Peripheral blood was obtained from EM patients, and the positive immune cell phenotype was confirmed using flow cytometry.After P-value correction, our two-sample MR showed that CD28 on CD28+ DN (CD4-CD8-) had a suggestive causal relationship with EM (β =0.040, 95% CI =1.02-1.06, P =0.00029, PFDR = 0.1984). The results of the other two main methods were similar: Weighted median (OR =1.031, 95% CI =1.00-1.07, P =0.082); MR-Egger (OR =1.032, 95% CI =1.10-1.06, P =0.044). The flow cytometry results indicated that the expression level of CD28 on CD28+ DN (CD4-CD-8) was significantly increased in the ectopic intima of EM patients.Our study demonstrated a causal relationship between immune traits and EM, and the results were verified by clinical samples. The study may provide new biomarkers for the early diagnosis and immunotherapy of EM.© 2025 Mei et al.





 +添加评论
+添加评论






















































 膜杰作
膜杰作 Star Staining
Star Staining

















