分子别名(Synonym)
ERBB2,CD340,HER-2,neu,HER2,MLN19,NEU,NGL,TKR1
表达区间及表达系统(Source)
Biotinylated Human Her2, His,Avitag, premium grade (HE2-H82E2) is expressed from human 293 cells (HEK293). It contains AA Thr 23 - Thr 652 (Accession # P04626-1).
Predicted N-terminus: Thr 23
It is produced under our rigorous quality control system that incorporates a comprehensive set of tests including sterility and endotoxin tests. Product performance is carefully validated and tested for compatibility for cell culture use or any other applications in the early preclinical stage. When ready to transition into later clinical phases, we also offer a custom GMP protein service that tailors to your needs. We will work with you to customize and develop a GMP-grade product in accordance with your requests that also meets the requirements for raw and ancillary materials use in cell manufacturing of cell-based therapies.
Request for sequence
蛋白结构(Molecular Characterization)

This protein carries a polyhistidine tag at the C-terminus, followed by an Avi tag (Avitag™).
The protein has a calculated MW of 72.9 kDa. The protein migrates as 101 kDa±3 kDa under reducing (R) condition, and 94 kDa when calibrated against Star Ribbon Pre-stained Protein Marker under non-reducing (NR) condition (SDS-PAGE) due to glycosylation.
标记(Labeling)
Biotinylation of this product is performed using Avitag™ technology. Briefly, the single lysine residue in the Avitag is enzymatically labeled with biotin.
蛋白标记度(Biotinylation)
As determined by Quantitative ELISA binding assay against streptavidin.
内毒素(Endotoxin)
Less than 0.01 EU per μg by the LAL method.
宿主蛋白残留(Host Cell Protein)
<0.5 ng/µg of protein tested by ELISA.
宿主核酸残留(Host Cell DNA)
<0.02 ng/μg of protein tested by qPCR.
无菌(Sterility)
Negative
支原体(Mycoplasma)
Negative.
纯度(Purity)
>95% as determined by SDS-PAGE.
>90% as determined by SEC-MALS.
制剂(Formulation)
Lyophilized from 0.22 μm filtered solution in PBS, pH7.4 with trehalose as protectant.
Contact us for customized product form or formulation.
重构方法(Reconstitution)
Please see Certificate of Analysis for specific instructions.
For best performance, we strongly recommend you to follow the reconstitution protocol provided in the CoA.
存储(Storage)
For long term storage, the product should be stored at lyophilized state at -20°C or lower.
Please avoid repeated freeze-thaw cycles.
This product is stable after storage at:
- -20°C to -70°C for 24 months in lyophilized state;
- -70°C for 12 months under sterile conditions after reconstitution.
质量管理控制体系(QMS)
电泳(SDS-PAGE)

Biotinylated Human Her2, His,Avitag, premium grade on SDS-PAGE under reducing (R) and non-reducing (NR) conditions. The gel was stained with Coomassie Blue. The purity of the protein is greater than 95% (With Star Ribbon Pre-stained Protein Marker).
SEC-MALS
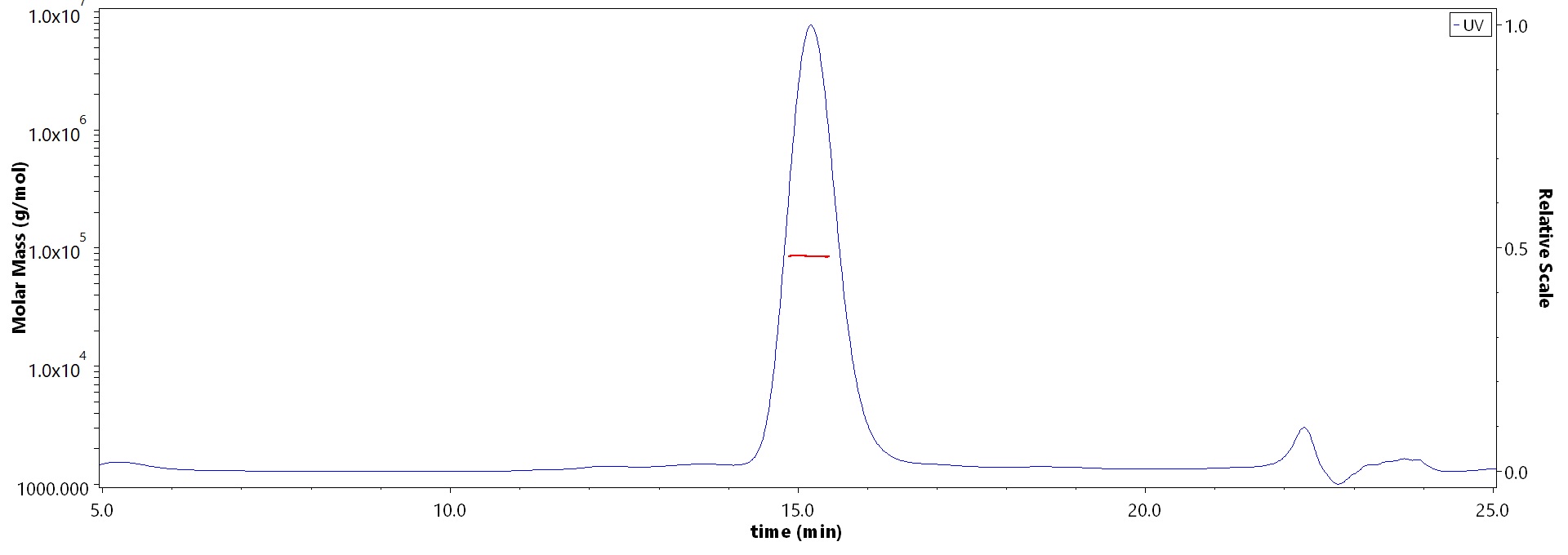
The purity of Biotinylated Human Her2, His,Avitag, premium grade (Cat. No. HE2-H82E2) is more than 90% and the molecular weight of this protein is around 75-110 kDa verified by SEC-MALS.
Report
活性(Bioactivity)-FACS

2e5 of anti-Her2 CAR-293 cells were stained with 100 μL of 1 μg/mL of Biotinylated Human Her2, His,Avitag, premium grade (Cat. No. HE2-H82E2) and negative control protein respectively, washed and then followed by PE-SA and analyzed with FACS (QC tested).
Protocol
活性(Bioactivity)-ELISA
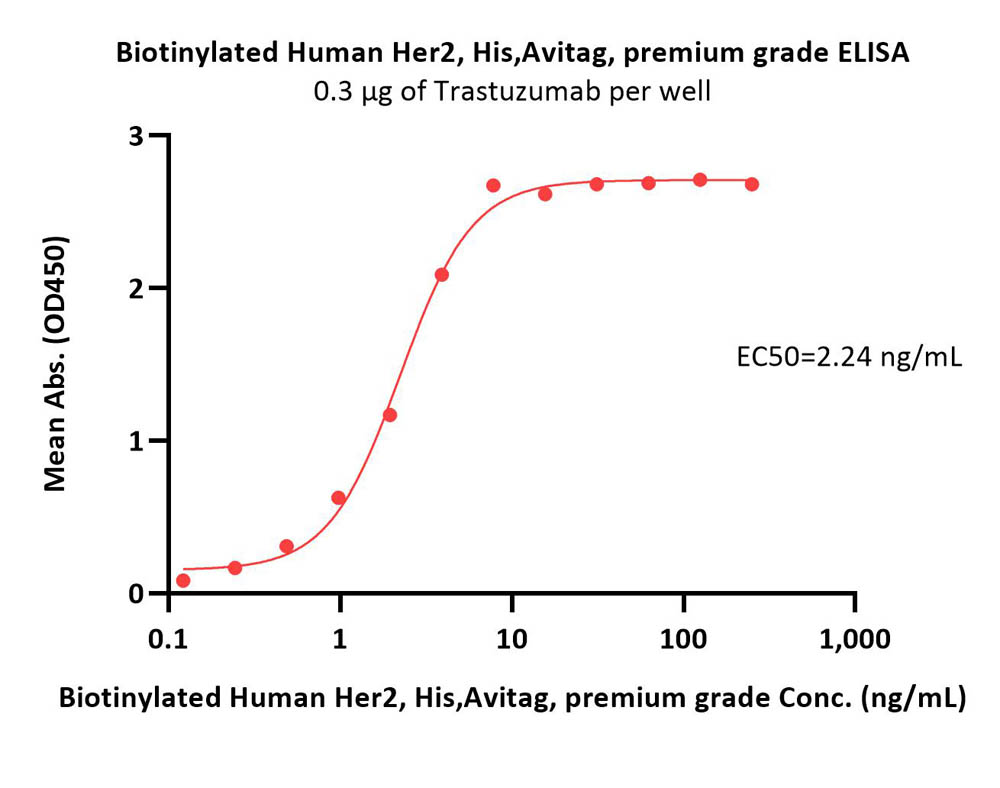
Immobilized Biotinylated Human Her2, His,Avitag, premium grade (Cat. No. HE2-H82E2) at 1 μg/mL (100 μL/well) on streptavidin (Cat. No. STN-N5116) precoated (0.5 μg/well) plate can bind Pertuzumab Biosimilar with a linear range of 0.2-8 ng/mL (QC tested).
Protocol
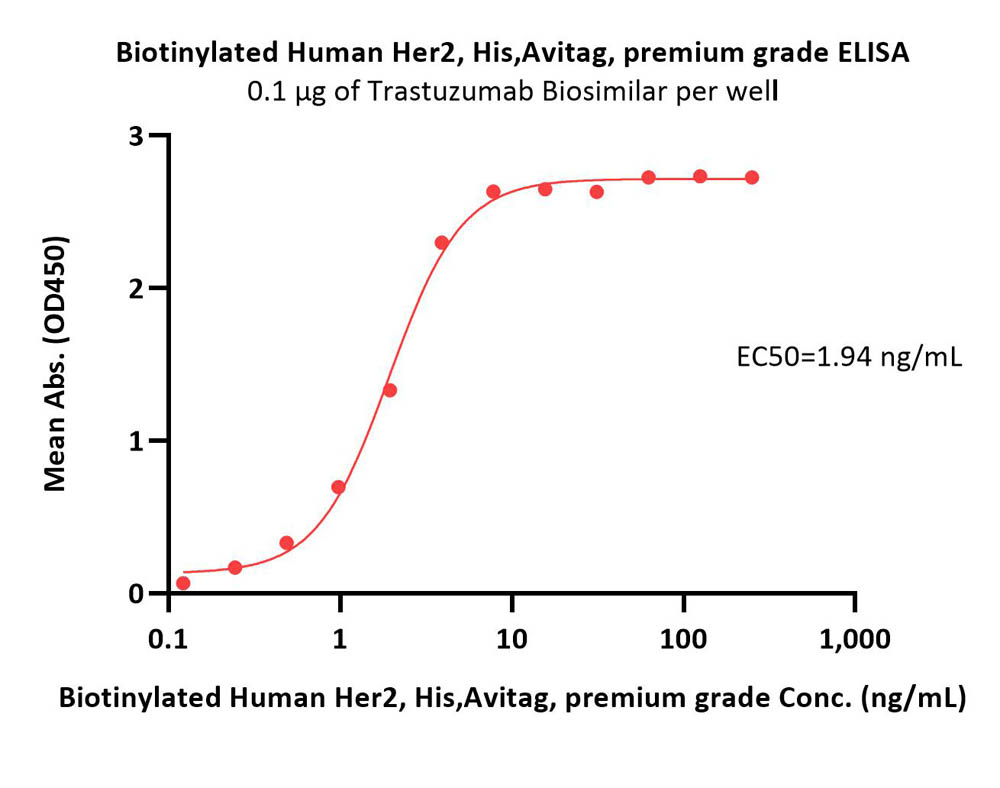
Immobilized Biotinylated Human Her2, His,Avitag, premium grade (Cat. No. HE2-H82E2) at 1 μg/mL (100 μL/well) on streptavidin (Cat. No. STN-N5116) precoated (0.5 μg/well) plate can bind Trastuzumab Biosimilar with a linear range of 0.2-2 ng/mL (Routinely tested).
Protocol
活性(Bioactivity)-SPR
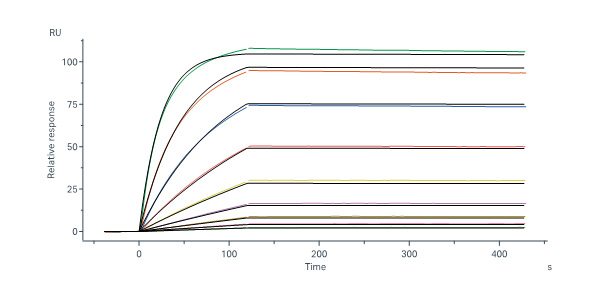
Trastuzumab Biosimilar captured on Protein A Chip can bind Biotinylated Human Her2, His,Avitag, premium grade (Cat. No. HE2-H82E2) with an affinity constant of 7.49 nM as determined in a SPR assay (Biacore 8K) (QC tested).
Protocol
 +添加评论
+添加评论背景(Background)
Human Epidermal growth factor Receptor 2 (HER2) is also called ERBB2, HER-2,HER-2 /neu, NEU, NGL,TKR1 and c-erb B2,and is a protein giving higher aggressiveness in breast cancers. It is a member of the ErbB protein family, more commonly known as the epidermal growth factor receptor family. HER2 is a cell membrane surface-bound receptor tyrosine kinase and is normally involved in the signal transduction pathways leading to cell growth and differentiation. HER2 is thought to be an orphan receptor, with none of the EGF family of ligands able to activate it. Approximately 30% of breast cancers have an amplification of the HER2 gene or overexpression of its protein product. Overexpression of this receptor in breast cancer is associated with increased disease recurrence and worse prognosis. HER2 appears to play roles in development, cancer, communication at the neuromuscular junction and regulation of cell growth and differentiation .























































 膜杰作
膜杰作 Star Staining
Star Staining















