分子别名(Synonym)
IgG2B,IgG2b Fc
表达区间及表达系统(Source)
Llama IgG2b Fc, Tag Free (IGB-L5204) is expressed from human 293 cells (HEK293). It contains AA Glu 1-Ser 243 (Accession # AAX73259.1).
Predicted N-terminus: Glu 1
Request for sequence
蛋白结构(Molecular Characterization)

This protein carries no "tag".
The protein has a calculated MW of 27.3 kDa. The protein migrates as 35-40 kDa under reducing (R) condition (SDS-PAGE) due to glycosylation.
内毒素(Endotoxin)
Less than 1.0 EU per μg by the LAL method.
纯度(Purity)
>90% as determined by SDS-PAGE.
>95% as determined by SEC-MALS.
制剂(Formulation)
Lyophilized from 0.22 μm filtered solution in Tris with Glycine, Arginine and NaCl, pH7.5. Normally trehalose is added as protectant before lyophilization.
Contact us for customized product form or formulation.
重构方法(Reconstitution)
Please see Certificate of Analysis for specific instructions.
For best performance, we strongly recommend you to follow the reconstitution protocol provided in the CoA.
存储(Storage)
For long term storage, the product should be stored at lyophilized state at -20°C or lower.
Please avoid repeated freeze-thaw cycles.
This product is stable after storage at:
- -20°C to -70°C for 12 months in lyophilized state;
- -70°C for 3 months under sterile conditions after reconstitution.
质量管理控制体系(QMS)
电泳(SDS-PAGE)
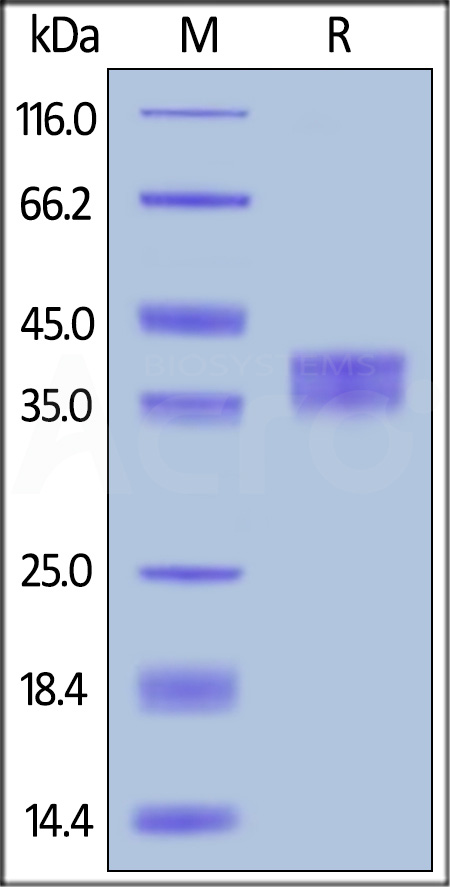
Llama IgG2b Fc, Tag Free on SDS-PAGE under reducing (R) condition. The gel was stained with Coomassie Blue. The purity of the protein is greater than 90%.
SEC-MALS
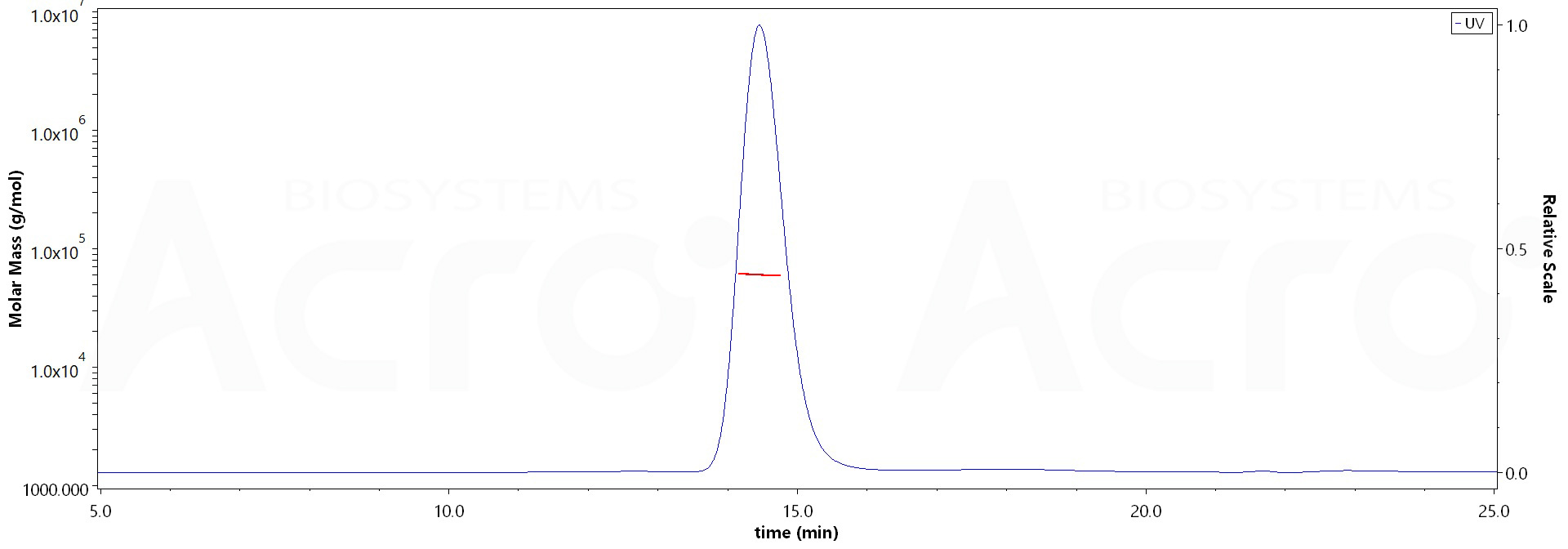
The purity of Llama IgG2b Fc, Tag Free (Cat. No. IGB-L5204) is more than 95% and the molecular weight of this protein is around 53-65 kDa verified by SEC-MALS.
Report
背景(Background)
Immunoglobulin G2 (IgG2) is a member of many immunoglobulin G developed and secreted by effective B cells. In wake of cutting by pepsin, IgG is divided into two F(ab)s with one antigen binding site and a high conserved Fc segment. The Fc segment bears a highly conserved N-glycosylation site. There are two members of IgG2: IgG2a and IgG2b. It was found that IgG2a was superior to IgG1 in activating complement. The glycosylation of the circulating immunoglobulin-γ (IgG) antibody molecules changes in rheumatoid arthritis. Ig gamma-2 chain Fc region contains two constant regions of IgG2 H chain (CH2, CH3).























































 膜杰作
膜杰作 Star Staining
Star Staining















