A phase 1, first-in-human, dose-escalation, expansion trial of cytokine encoding synthetic mRNA-mixture alone or with cemiplimab in advanced solid tumorsBechter, Loquai, Champiat
et alClin Cancer Res (2025)
Abstract: We investigated SAR441000 (mixture of four mRNAs encoding interleukin [IL]-12, single chain interferon [IF]-α-2b, granulocyte-macrophage colony-stimulating factor, and IL-15 sushi domain) alone or in combination with cemiplimab in patients with advanced solid tumors.SAR441000 was intratumorally administered weekly in a 4-week cycle in monotherapy and in a 3-week cycle at a pre-defined dose level (DL) with 350 mg cemiplimab (intravenously) every 3 weeks in combination therapy. The primary objective was to determine maximum tolerated or maximum administered dose (MAD), overall safety, tolerability, and objective response rate of SAR441000.We enrolled 77 patients previously treated with anti-cancer therapies (escalation monotherapy: N=21; escalation combination: N=15; and expansion combination [PD-1 refractory melanoma]: N=41). MAD at DL8 was 4000 µg. The most common Grade ≥3 treatment-related adverse event was fatigue in escalation phase (monotherapy: 28.6%; combination therapy: 66.7%) and injection-site pain (31.7%) in expansion phase. In combination therapy, one patient in escalation and two in expansion phase achieved partial responses. At 4000 μg (highest dose) across all cohorts, the maximum fold change in plasma cytokine concentration was the highest and lowest for IFN-α-2 (74.9-folds) and IL-15 (1.96-folds), respectively. Increased blood IFN-γ and IP-10 levels were observed for most patients.Intratumoral administration of SAR441000 in combination with cemiplimab, was generally well tolerated with anti-tumor activity in loco-regional disease setting. Anecdotal evidence of pharmacodynamic immune-modulatory effect and distant non-injected lesion anti-tumor response was observed, without significant effect in patients with advanced solid tumors previously treated with anti-PD1 therapies.
Improvement of RSV-Induced Asthma in Mice: A Study Based on Icariin-Mediated PD-1Fu, Wang
Front Biosci (Landmark Ed) (2025) 30 (3), 26061
Abstract: Infection with respiratory syncytial virus (RSV) has the potential to exacerbate asthma by causing prolonged inflammation in the airways. Mounting evidence has revealed the significant involvement of programmed cell death protein-1 (PD-1) in the development of asthma. Although icariin (IC) has shown potential in improving airway remodeling in ovalbumin (OVA)-induced asthma, its impact and underlying mechanisms in cases of asthma aggravated by RSV infection are not thoroughly understood.To explore the effect of IC on RSV-infected asthmatic mice and the mechanism involving PD-1.A model of asthmatic mice infected with RSV was developed. To evaluate the effects of IC treatment, general behavioral characterization, histopathologic analysis, bronchoalveolar lavage fluid (BALF) analysis, and enzyme-linked immunosorbent assays (ELISA) were performed to assess the frequency of sneezing and nose scratching, the content of OVA-specific IgE, oxidative stress and airway inflammation in mice. Apoptosis was also assessed by terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL). Finally, the expression levels of apoptosis protein, oxidative stress-related protein, and PD-1 were assessed by western blot.IC significantly ameliorated the sneezing and nose-scratching frequency (p < 0.001) and decreased OVA-specific IgE levels in asthmatic mice infected with RSV (p < 0.01). IC treatment remarkably reduced the infiltration of inflammatory cells around the alveoli and lowered the overall inflammation score. It also notably decreased the levels of inflammatory cytokines interleukin-4 (IL-4), IL-13, and IL-5, and decreased the numbers of neutrophils, eosinophils, and macrophages in the bronchoalveolar lavage fluid (BALF) (p < 0.001). IC ameliorated oxidative stress in RSV-infected asthmatic mice (p < 0.001). In addition, IC reduced apoptosis while increasing PD-1 expression in asthmatic mice infected with RSV (p < 0.001). Interestingly, si-PD-1 significantly reversed IC inhibition of inflammatory cytokines and apoptosis-related proteins, and promoted PD-1 protein expression (p < 0.01). The findings suggested that IC might be effective in alleviating asthma triggered by RSV in mice by regulating the expression of PD-1.IC ameliorated RSV-induced asthma in mice by regulating PD-1 expression, and may hold promise as a potential therapeutic agent for RSV-induced asthma in mice. These findings provide valuable insights into the possibility of using IC as a treatment option for asthma caused by RSV.© 2025 The Author(s). Published by IMR Press.
Recent developments on checkpoint inhibitors, CAR T cells, and beyond for T cell-based immunotherapeutic strategies against cancerAli, Arshad, Summer
et alJ Oncol Pharm Pract (2025)
Abstract: ObjectiveThere was a dire need to construct a review of the recent developments on Immune checkpoint inhibitors (ICIs), CAR T Cells, and other approaches for T cell-based immunotherapeutic strategies against cancer as cancer has become one of the most fatal diseases that is responsible for causing several deaths per annum.Data sourcesMultiple published data was acquired from the high-impact factor journal articles.Data summaryMultiple clinical strategies have been in use today such as radiotherapy, chemotherapy and immunotherapy to treat cancer of different types. Among novel cancer management strategies, the role of cancer immunotherapy by T cells has become immensely important. Cancer immunotherapy has revolutionized treatment approaches and it basically utilizes the body's immune system to treat cancer. At the forefront of this revolution, T cells are considered as the fundamental components of immune system.ConclusionsThe current review explores the therapeutic potential of T cells in the fight against cancer by applying strategies such as various ICIs (PD-1/PD-L1, CTLA-4, TIGIT, BTLA, TIM3, LAG3) and adoptive cell therapy. ICIs stimulate the body's existing anti-tumor T-cell response by the way of removing immune system inhibitors. On the other hand, in adoptive cell therapy (ACT) patient's T cells are modified to identify and attack tumor cells. Furthermore, this review also highlights significant successes that are observed with these therapies, notably PD-1 blockade and CAR T-cell therapy for various tumors. Moreover, this review also explores the potential of therapeutic vaccination, bispecific antibodies and cytokine therapy to enhance the antitumor activity. Therapeutic vaccines expose immune system to various tumor-associated antigens and training it to identify and then attack cancer cells, showing promising results in different types of cancers such as prostate cancer and melanoma. While, cytokine therapy is accompanied by the use of cytokines such as interleukin-2 (IL-2) to stimulate immune cell activity and proliferation, thereby boosting the overall anti-tumor immune response. Lastly, the current review explores the promising future of T cell-based immunotherapy, envisioning advancements in CAR design and gene editing techniques that can enhance efficacy across a broader spectrum of cancers.
PD-1 inhibitors improve the efficacy of tyrosine kinase inhibitors combined with transcatheter arterial chemoembolization in advanced hepatocellular carcinoma: a meta-analysis and trial sequential analysisYu, Li, Yang
et alScand J Gastroenterol (2025)
Abstract: This meta-analysis and trial sequential analysis (TSA) aimed to evaluate the efficacy and safety of triple therapy with tyrosine kinase inhibitors (TKIs) combined with transcatheter arterial chemoembolization (TACE) plus programmed death 1 (PD-1) inhibitors (T-T-P) and dual therapy with TKIs combined with TACE (T-T) for the treatment of advanced unresectable hepatocellular carcinoma (uHCC).Literature related to the efficacy of TKIs combined with TACE plus PD-1 inhibitors in uHCC was searched using the Embase, PubMed, and Cocrane libraries. TSA was used to reduce false positive results due to random error.Seventeen articles were included in this meta-analysis, including 2,561 patients. In the T-T-P group, OS [HR 0.45, 95% confidence interval (CI) 0.39-0.52; p = 0.000], PFS [HR 0.43, 95% CI 0.38 - 0.48; p = 0.000], were significantly prolonged compared to those in the T-T group; ORR (RR 1.59 [95% CI 1.39-1.81]; p = 0.000) and DCR (RR 1.26 [95% CI 1.15-1.37]; p = 0.000) were significantly higher. TSA analysis showed early results without further testing. Prognostic factor analysis demonstrated that portal vein tumor thrombus (PVTT) and extrahepatic metastasis were common independent risk factors for OS and PFS. Regarding grade 3/4 adverse events results showed no statistically significant differences in any of them.Compared with T-T treatment group, the T-T-P treatment group exhibited a notable improvement in OS and PFS, particularly in cases of PVTT and extrahepatic metastasis. Furthermore, it can markedly enhance the ORR and DCR in patients with uHCC.

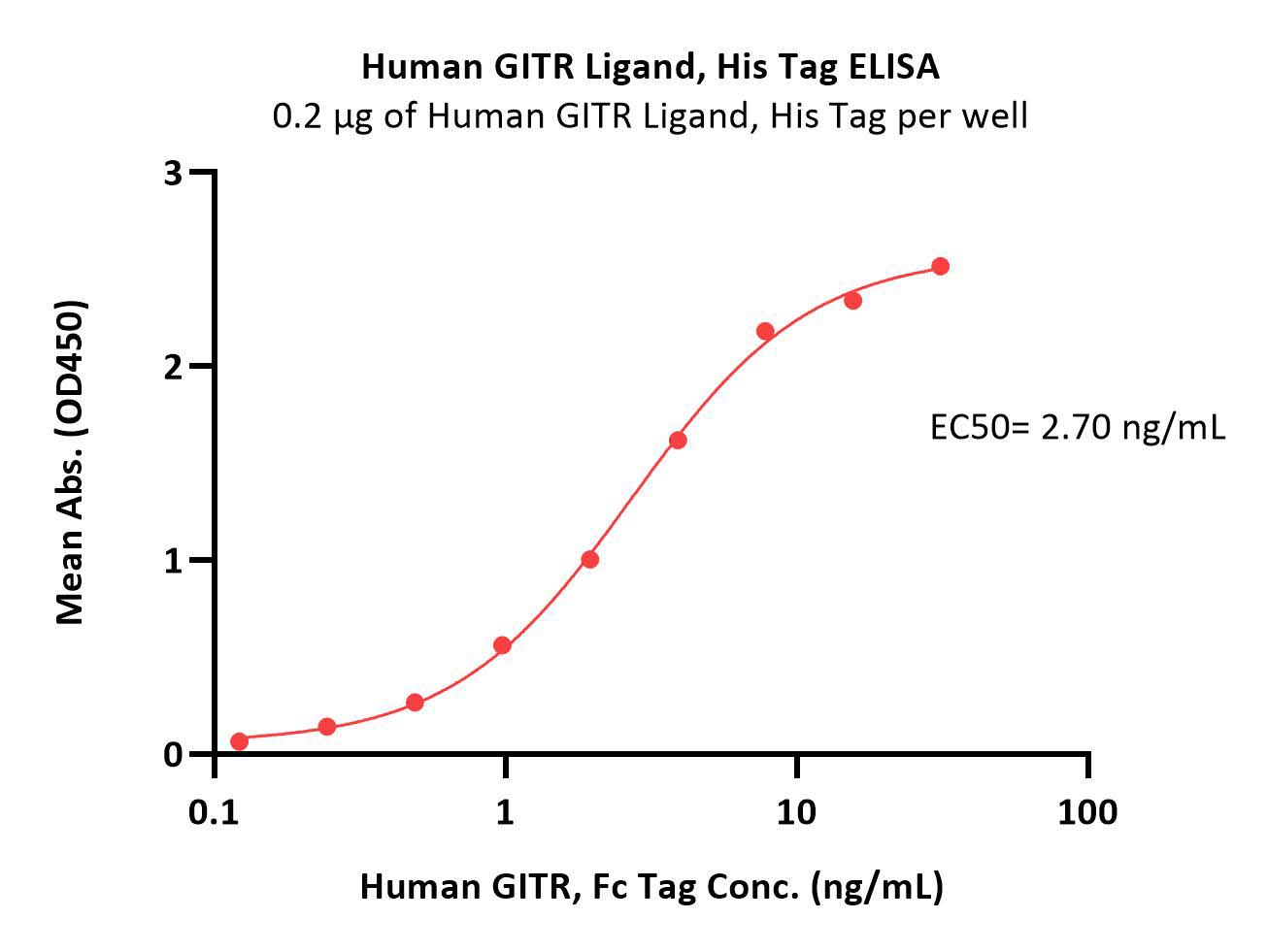


 >
>























































 膜杰作
膜杰作 Star Staining
Star Staining


















