Black Pigmentation on the Tongue Induced by Long-Term Use of Tetracycline Antibiotics in a Colorectal Cancer Patient With Epidermal Growth Factor Receptor (EGFR) Inhibitor-Associated Skin Lesions: A Case Report and Literature ReviewInagaki, Ichimura, Ichikura
et alCureus (2025) 17 (2), e79261
Abstract: Skin lesions are prevalent in patients with metastatic colorectal cancer (mCRC) receiving epidermal growth factor receptor (EGFR) inhibitors, such as panitumumab and cetuximab, often necessitating long-term management. Despite adequate treatment involving topical adrenocorticosteroid medications, severe skin lesions may result in prolonged or discontinued EGFR inhibitor administration. Consequently, tetracycline antibiotics, with their antibacterial and anti-inflammatory effects, are recommended for EGFR inhibitor-induced acne dermatitis. We present a 68-year-old male patient with mCRC who underwent chemotherapy with panitumumab, folinic acid, 5-fluorouracil, and irinotecan but subsequently exhibited black pigmentation on the tongue due to six months of prolonged minocycline administration for treating panitumumab-induced skin lesions. Suspecting minocycline-related adverse effects based on the description and a score of 7 on the Naranjo scale, minocycline was discontinued while other medications were maintained. The color of the dorsal tongue gradually normalized within two weeks after discontinuing minocycline. Presently, chemotherapy is continued, whereas panitumumab is repeatedly started and stopped according to the severity of acneiform efflorescence and paronychia. Panitumumab was continued without dose reduction or discontinuation owing to the suppression of skin lesions by minocycline; however, the patient developed black pigmentation on the tongue accompanied by dysgeusia, negatively affecting the quality of life. Discontinuing minocycline resulted in a gradual improvement of these symptoms. This report underscores the importance of clinicians being vigilant to the risk of black pigmentation on the tongue in patients receiving long-term tetracycline antibiotics for the treatment of EGFR inhibitor-induced skin lesions.Copyright © 2025, Inagaki et al.
Practical Consensus Guidelines on the Use of Cetuximab in Head and Neck Squamous Cell Carcinoma (HNSCC)Parikh, Biswas, Dhamne
et alSouth Asian J Cancer (2025) 14 (1), 90-102
Abstract: Head and neck squamous cell carcinoma (HNSCC) is the most common malignancy group in India and several other low- and middle-income countries. Currently, majority of the patients present in advanced stage where systemic therapy is standard of care. Multiple relapses are also not uncommon. Almost all HNSCC tumors have epidermal growth factor receptor (EGFR) overexpression, making an attractive target. Cetuximab is the most successful method to target EGFR in HNSCC. After decades of its use, it still is a prominent part of the current management guidelines. Since other agents have also been proven to be useful, we felt it was necessary to develop a real-world consensus guideline to help the decision-making process among the community oncologists. Our expert committee therefore put together currently available data, insights from their real-world clinical practice, and voted to arrive at a consensus. These consensus guidelines represent how cetuximab should be used today in the management of HNSCC.MedIntel Services Pvt Ltd. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. ( https://creativecommons.org/licenses/by-nc-nd/4.0/ ).
Colorectal Cancer: Risk Factors, Novel Approaches in Molecular Screening and TreatmentAnbari, Ghanadi
Int J Mol Cell Med (2025) 14 (1), 576-605
Abstract: By 2040 the burden of colorectal cancer will increase to 3.2 million new cases per year and 1.6 million deaths per year. This highlights the importance of improving preventive measures and treatment strategies. This piece concisely overviews the latest therapeutic and diagnostic approaches for colorectal cancer. In 2019, factors such as low milk intake, smoking, insufficient calcium consumption, and alcohol use had a significant impact on colorectal cancer DALYs worldwide. A comprehensive search was conducted in December 2023 using keywords related to drugs, therapeutic agents, colorectal cancer, diagnostic methods, epidemiology, and novel therapeutic approaches in the PubMed and Scopus databases. Initially, 325 articles were identified based on titles, abstracts, and publication dates. After removing duplicates, 170 unique articles were included. Medications like Nimotuzumab, Cetuximab, and Panitumumab target the Epidermal Growth Factor Receptor (EGFR), which EGF activates. HER2, activated by ligands, is the focus of drugs like Trastuzumab and Pertuzumab. The PD-1/PD-L1 and CTLA-4 pathways, as the immune checkpoints, which involve T cells, are targeted by medications like Ipilimumab. Adoptive cell therapy, including CAR-T cell therapy, TCR modification, and enhancing T cell activity through tumor-infiltrating lymphocytes, is used to combat cancer cell growth. In medical advancements, adoptive cell transfer therapy (ACT) and exosomes in the tumor immune microenvironment (TME) are notable treatment methods that boost the immune system. HIF1A-AS1, CRNDE-h, NEAT1, ZFAS1, and GAS5, along with IGFBP-2, have demonstrated significant CRC diagnostic capacity. Compared to CRC patients with low HIF1A-AS1 expression, individuals with high expression levels were linked to a worse 5-year survival rate.© The Author(s).
EGFR-mediated local invasiveness and response to Cetuximab in head and neck cancerZhou, He, Zhao
et alMol Cancer (2025) 24 (1), 94
Abstract: Recurrent/metastatic head and neck squamous cell carcinoma (R/M-HNSCC) is a severe, frequently lethal condition. Oncogene addiction to epidermal growth factor receptor (EGFR) is a hallmark of HNSCC, but the clinical efficacy of EGFR-targeted therapies remains low. Understanding molecular networks governing EGFR-driven progression is paramount to the exploration of (co)-treatment targets and predictive markers.We performed function-based mapping of differentially expressed genes in EGFR-mediated local invasion (fDEGs) using photoconvertible tracers and RNA-sequencing (RNA-seq) in a cellular 3D-model.Upon alignment with public single-cell RNA-seq (scRNA-seq) datasets and HNSCC-specific regulons, a gene regulatory network of local invasion (invGRN) was inferred from gene expression data, which was overrepresented in budding tumors. InvGRN comprises the central hubs inhibin subunit beta alpha (INHBA) and snail family transcriptional repressor 2 (SNAI2), and druggable fDEGs integrin subunit beta 4 (ITGB4), laminin 5 (LAMB3/LAMC2), and sphingosine kinase 1 (SPHK1). Blockade of INHBA repressed local invasion and was reverted by activin A, laminin 5, and sphingosine-1-phosphate, demonstrating a functional interconnectivity of the invGRN. Epithelial-to-mesenchymal transition (EMT) of malignant cells and the invGRN are induced by newly defined EGFR-activity subtypes with prognostic value that are promoted by amphiregulin (AREG) and epiregulin (EREG). Importantly, co-inhibition of SPHK1 showed synthetic effects on Cetuximab-mediated invasion blockade and high expression of selected fDEGs was associated with response to Cetuximab in patient-derived xenotransplantation (PDX) and R/M-HNSCC patients.We describe an actionable network of EGFR-mediated local invasion and define druggable effectors with predictive potential regarding the response of R/M-HNSCC to Cetuximab.© 2025. The Author(s).
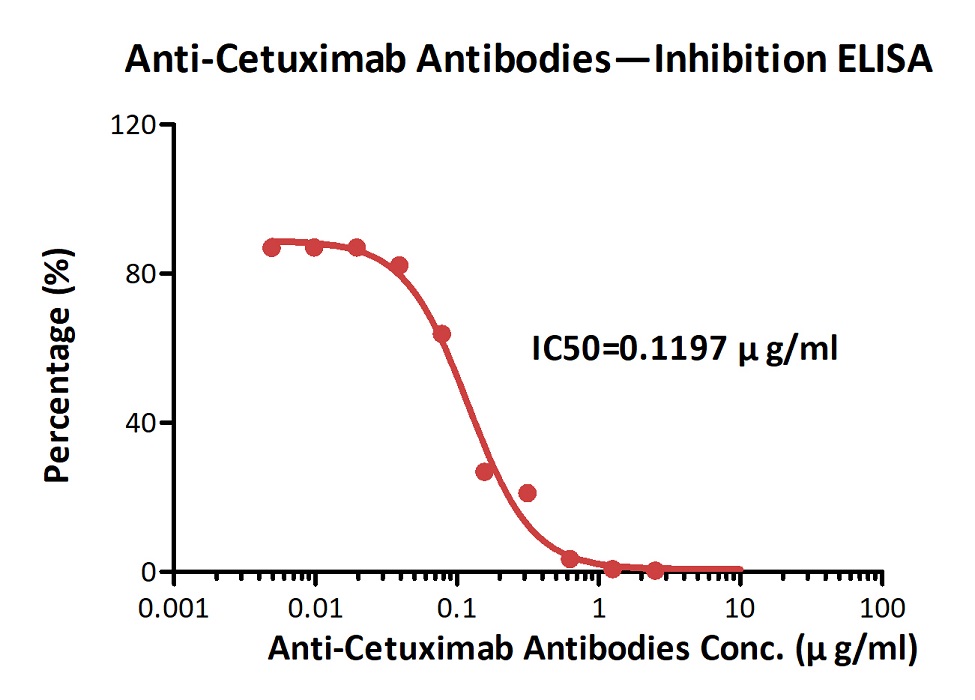
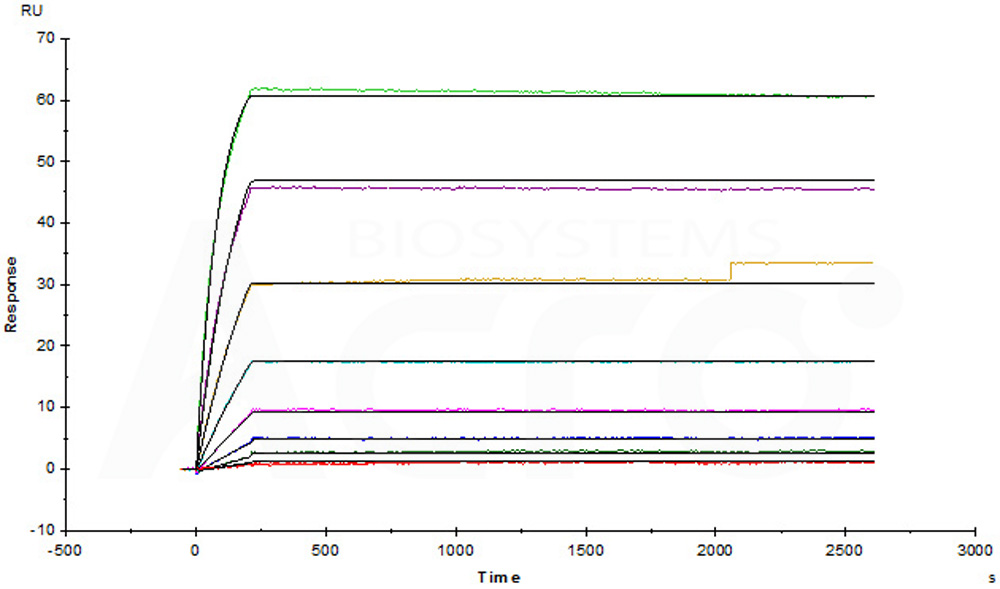























































 膜杰作
膜杰作 Star Staining
Star Staining















