Kupffer cell expression of macrophage receptor with collagenous structure (MARCO) modulates macrophage gene induction and limits acute liver injuryPoole, Wei, Schulte
et alToxicol Sci (2025)
Abstract: Macrophages displaying a pro-repair and anti-inflammatory polarization have been implicated in resolution of acute liver injury. Macrophage receptor with collagenous structure (MARCO) expression marks tolerogenic hepatic macrophages and is expressed by pro-resolution macrophages in the injured liver. We tested the hypothesis that MARCO promotes repair of the acetaminophen (APAP)-injured liver. Robust and sustained induction of MARCO mRNA and protein expression was evident in livers of mice challenged with a hepatotoxic dose of APAP (ie, 300 mg/kg), whereas hepatic MARCO induction failed in mice with APAP-induced liver failure (ie, 600 mg/kg). Serum proteomics identified a significant increase in serum MARCO levels in surviving acute liver failure (ALF) patients, but not in ALF patients who died. MARCO expression was high in F480+ liver macrophages, and MARCO deficiency reduced macrophage expression of pro-resolution markers such as Gpnmb and Mertk during the repair phase (ie, 48 hours). The results suggested a delay in necrosis resolution along with a trend towards increased mortality in APAP-challenged MARCO-/- mice. Notably, a robust increase in peak hepatic injury (ie, 6-24 hours post-APAP challenge) was evident in MARCO-/- mice, which could not be ascribed to differences in NAPQI/APAP-adduct generation nor changes in hepatic neutrophil/macrophage numbers. Interestingly, a reduction in hepatic CD11c+ cells, shown previously to limit APAP-induced liver injury, was evident 24 hours after APAP challenge in MARCO-/- mice. The results indicate that MARCO deficiency worsens APAP-induced acute liver injury in mice and provide experimental and initial translational evidence linking MARCO induction to positive outcomes in acute liver injury.© The Author(s) 2025. Published by Oxford University Press on behalf of the Society of Toxicology. All rights reserved. For commercial re-use, please contact reprints@oup.com for reprints and translation rights for reprints. All other permissions can be obtained through our RightsLink service via the Permissions link on the article page on our site—for further information please contact journals.permissions@oup.com.
SIRPα modulates microglial efferocytosis and neuroinflammation following experimental subarachnoid hemorrhage via the SHP1/STAT6 axisZhang, Zou, Tang
et alJ Neuroinflammation (2025) 22 (1), 88
Abstract: Subarachnoid hemorrhage induces extensive neuronal cell death, leading to the release of damage-associated molecular patterns (DAMPs). These DAMPs, along with hemoglobin and cell corpses, trigger localized inflammation. Signal regulatory protein alpha (SIRPα) plays a crucial role in efferocytosis by acting as a "don't eat-me" signal, modulating inflammation and tissue homeostasis. However, the precise function and regulatory mechanisms of SIRPα in efferocytosis remain unclear. Proteomic analysis of cerebrospinal fluid (CSF) reveals that SIRPα levels are significantly elevated in the CSF of SAH patients and correlate with clinical outcomes. In vivo and in vitro studies show that microglial knockdown of SIRPα promotes efferocytosis and attenuates neuroinflammation following SAH. SIRPα inhibits efferocytosis by recruiting and phosphorylating SHP1 and SHP2 through phosphorylation of four tyrosine residues in its cytoplasmic domain, with SHP1 playing a particularly critical role. Mutation of these tyrosine residues to non-phosphorylatable alanine residues enhances efferocytosis and reduces neuroinflammation in vitro. RNA-seq analysis suggests that this mutation upregulates the expression of "eat-me" signals, MerTK and CD36, and identifies STAT6 as a key transcription factor involved in this process. In conclusion, SIRPα plays a central role in regulating microglia efferocytosis and neuroinflammation after SAH via the SHP1/STAT6 axis. Targeting this pathway may provide a promising therapeutic approach for SAH.© 2025. The Author(s).
Nanosecond laser induces proliferation and improved cellular health within the retinal pigment epitheliumJobling, Findlay, Greferath
et alFront Med (Lausanne) (2025) 12, 1516900
Abstract: Age-related macular degeneration (AMD) is a leading cause of vision loss in those over 60 years of age. Although there are limited interventions that may prevent the development or progression of disease, more efficacious treatments are required. Short-pulsed laser treatment shows promise in delaying progression of early disease. This work details how nanosecond laser influences the retinal pigment epithelium (RPE), the principal cell type implicated in AMD.C57BL/6J mice (3-month-old) underwent monocular nanosecond laser treatment to assess short-term RPE response, while 9-month-old C57BL/6J and ApoEnull mice were similarly treated and longer-term responses investigated after 3 months. Human tissue was also obtained after 2 nanosecond laser treatments (1 month apart). RPE proliferation was assessed using bromodeoxyuridine and RPE gene change explored using qPCR and RNAseq. Melanin and lipofuscin content were quantified using histological techniques.Nanosecond laser induced RPE proliferation in treated and fellow mouse eyes, with monolayer repair occurring within 3 days. This was replicated in human tissue, albeit over a longer duration (1-4 weeks). Wildtype animals showed no overt change in RPE gene expression after short or longer post-treatment durations, while laser treated ApoEnull animals showed increased Mertk and Pedf expression, and a reduced number of dysregulated aging genes in treated and fellow eyes after 3 months. Furthermore, melanin and lipofuscin content were restored to wildtype levels in laser-treated ApoEnull RPE, while melanolipofuscin granules were reduced within treated regions of human RPE.This work shows nanosecond laser stimulates RPE proliferation and results in an improved cellular phenotype. These data provide a biological basis for the prophylactic use of nanosecond lasers in AMD.Copyright © 2025 Jobling, Findlay, Greferath, Vessey, Gunnam, Morrison, Venables, Guymer and Fletcher.
Identification of glycolysis-related gene signatures for prognosis and therapeutic targeting in idiopathic pulmonary fibrosisGao, Sun, Hu
et alFront Pharmacol (2025) 16, 1486357
Abstract: Glycolysis plays a crucial role in fibrosis, but the specific genes involved in glycolysis in idiopathic pulmonary fibrosis (IPF) are not well understood.Three IPF gene expression datasets were obtained from the Gene Expression Omnibus (GEO), while glycolysis-related genes were retrieved from the Molecular Signatures Database (MsigDB). Differentially expressed glycolysis-related genes (DEGRGs) were identified using the "limma" R package. Diagnostic glycolysis-related genes (GRGs) were selected through least absolute shrinkage and selection operator (LASSO) regression regression and support vector machine-recursive feature elimination (SVM-RFE). A prognostic signature was developed using LASSO regression, and time-dependent receiver operating characteristic (ROC) curves were generated to evaluate predictive performance. Single-cell RNA sequencing (scRNA-seq) data were analyzed to examine GRG expression across various cell types. Immune infiltration analysis, Gene Set Enrichment Analysis (GSEA), and Gene Set Variation Analysis (GSVA) were performed to elucidate potential molecular mechanisms. A bleomycin (BLM)-induced pulmonary fibrosis mouse model was used for experimental validation via reverse transcription-quantitative polymerase chain reaction (RT-qPCR).14 GRGs (VCAN, MERTK, FBP2, TPBG, SDC1, AURKA, ARTN, PGP, PLOD2, PKLR, PFKM, DEPDC1, AGRN, CXCR4) were identified as diagnostic markers for IPF, with seven (ARTN, AURKA, DEPDC1, FBP2, MERTK, PFKM, SDC1) forming a prognostic model demonstrating predictive power (AUC: 0.831-0.793). scRNA-seq revealed cell-type-specific GRG expression, particularly in macrophages and fibroblasts. Immune infiltration analysis linked GRGs to imbalanced immune responses. Experimental validation in a bleomycin-induced fibrosis model confirmed the upregulation of GRGs (such as AURKA, CXCR4). Drug prediction identified inhibitors (such as Tozasertib for AURKA, Plerixafor for CXCR4) as potential therapeutic agents.This study identifies GRGs as potential prognostic biomarkers for IPF and highlights their role in modulating immune responses within the fibrotic lung microenvironment. Notably, AURKA, MERTK, and CXCR4 were associated with pathways linked to fibrosis progression and represent potential therapeutic targets. Our findings provide insights into metabolic reprogramming in IPF and suggest that targeting glycolysis-related pathways may offer novel pharmacological strategies for antifibrotic therapy.Copyright © 2025 Gao, Sun, Hu, Song, Liu, Zou, Zhu and Cheng.

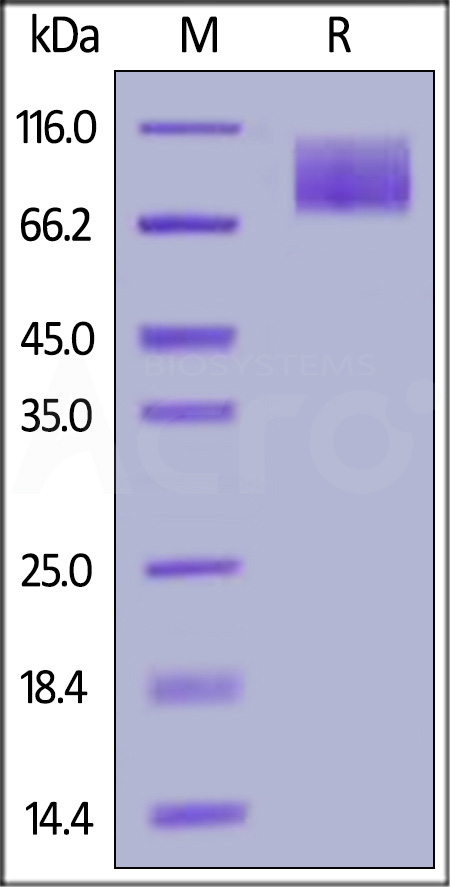
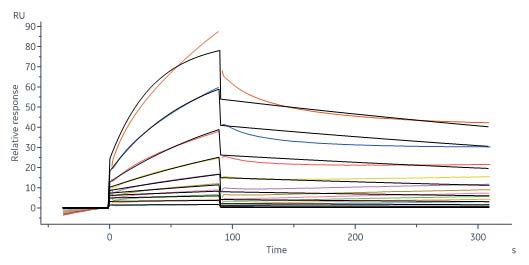























































 膜杰作
膜杰作 Star Staining
Star Staining















