Hyperbaric oxygen potentiates platelet-rich plasma composition and accelerates bone healingChang, Huang, Chen
et alJ Orthop Translat (2025) 51, 1-12
Abstract: This study aimed to investigate whether platelet-rich plasma (PRP) obtained from the blood of rats preconditioned with hyperbaric oxygen (HBOP) would enhance the biological activity of PRP and accelerate the healing process of femur fractures in a rat model.PRP was derived from blood samples of healthy rats subjected to either hyperbaric oxygen (hPRP) or normobaric air (nPRP). A closed femur fracture model was established in male Wistar rats, with treatments of hPRP or nPRP administered around the fracture site immediately post-fracture and on days 7, 14, 21, and 28. Growth factor concentrations in hPRP and nPRP were biochemically quantified. Bone healing was assessed weekly by X-ray, while histological and immunofluorescence analyses evaluated inflammatory status, osteoprotegerin (OPG), receptor activator of nuclear factor kappa-B ligand (RANKL) expression, and the presence of osteoblasts, osteoclasts, and osteocytes during healing. The effects of hPRP and nPRP on MC3T3-E1 preosteoblast migration and proliferation were also tested in vitro.hPRP showed significantly higher concentrations of growth factors such as activin-A, brain-derived neurotrophic factor, nerve growth factor, Flt-3 Ligand, granulocyte-macrophage colony-stimulating factor, hepatocyte growth factor, and platelet-derived growth factor, compared to nPRP. In vitro, hPRP demonstrated more significant effects on preosteoblast migration and proliferation. In vivo, hPRP treatment resulted in enhanced bone healing, higher OPG levels in osteoblasts and osteoclasts, and an elevated OPG/RANKL ratio compared to nPRP.HBOP enhances the biological activity of PRP and accelerates bone healing in a closed femur fracture model in rats. This study highlights the regenerative potential of PRP when preconditioned with hyperbaric oxygen for use in bone fracture therapy.PRP is widely used in treating bone defects and fractures, but its enhancement through HBOP remains underexplored. Our findings demonstrate that HBOP potentiates the biological activity of PRP, offering promising therapeutic potential for bone fracture healing.Enriching growth factors in PRP through HBOP could significantly improve tissue regeneration, especially in bone healing. The potential of hPRP in clinical applications is highly promising, particularly in orthopaedic surgery, trauma care, sports medicine, and managing bone healing in compromised patients.© 2024 The Authors.
Prognostic Factors, FLT-3 Mutations, and Treatment Outcomes with Pediatric-Inspired Protocols in Adolescent and Young Adults and Adult Patients with Acute Lymphoblastic LeukemiaOanunu, Gross Even-Zohar, Aumann
et alActa Haematol (2025)
Abstract: The treatment protocols of adolescent and young adult (AYA) patients with acute lymphoblastic leukemia (ALL) have evolved, with the advent of pediatric-based regimens, measurable residual disease monitoring, and mutation analysis. Among the latter, previous reports have identified FLT-3 mutations in up to 5% of pediatric patients; however, the full clinical significance of these mutations in the non-pediatric population is still uncertain.Our cohort includes AYA patients with ALL treated with the NY-II and BFM protocols at different time periods, allowing analysis of prognostic factors and survival outcomes. Additionally, we analyzed DNA samples for FLT-3 mutations, focusing on the potential prognostic implications and treatment responses within our cohort.No significant differences were found in overall survival or progression-free survival between the two treatment protocols. However, a higher rate of hematopoietic stem-cell transplantation was noted in the NY-II patients. Older age and high WBC count at presentation were identified as adverse prognostic factors using multivariate analysis. FLT-3 mutations were identified in 4 patients (5%) of the cohort, with only 1 patient having FLT-3 internal tandem duplication mutation and 3 patients having FLT-3-tyrosine kinase domain mutations.The low rate and variability of FLT-3 mutations in an Israeli cohort precludes broad conclusions regarding their prognostic significance. In our cohort, age and WBC count but not treatment protocol or FLT-3 mutations influenced survival.© 2025 The Author(s). Published by S. Karger AG, Basel.
A phase I study of MLN4924 and belinostat in relapsed/refractory acute myeloid leukemia or myelodysplastic syndromeMaher, Shafer, Schaar
et alCancer Chemother Pharmacol (2025) 95 (1), 24
Abstract: Relapsed and/or refractory acute myeloid leukemia and high-risk myelodysplastic syndrome continue to have a poor prognosis with limited treatment options despite advancements in rational combination and targeted therapies. Belinostat (an HDAC inhibitor) and Pevonedistat (a NEDD8 inhibitor) have each been independently studied in hematologic malignancies and have tolerable safety profiles with limited single-agent activity. Preclinical studies in AML cell lines and primary AML cells show the combination to be highly synergistic, particularly in high-risk phenotypes such as p53 mutant and FLT-3-ITD positive cells. Here, we present the safety, pharmacokinetics and pharmacodynamics of belinostat and pevonedistat in a dose escalation Phase I study in AML and High-Risk MDS.Eighteen patients (16 with AML, 2 with MDS) were treated at 5 dose levels (belinostat 800-1000 mg/m2, pevonedistat 20-50 mg/m2). Safety and tolerability were assessed according to protocol defined dose limiting toxicities (DLTs). Correlative pharmacokinetic and pharmacodynamic analyses were performed.No dose limiting toxicities were noted. Most Grade 3 or 4 toxicities were hematologic in nature. The best response was stable disease in four patients, and complete remission in one patient who qualified as an exceptional responder. Pharmakokinetic studies revealed no association between drug exposure and best response. Pharmacodynamic RT-PCR studies demonstrated post-treatment increases in several proteins, including quantitative increases in the oxidative stress protein NQO1, ferroptosis protein SLC7A11, and GSR, linked to glutathione metabolism and oxidative stress, as did the anti-oxidants SRXN1 and TXNRD1.Patterns of post-treatment changes in correlative pharmacodynamic parameters may suggest possible mechanistic changes in the DNA damage response, oxidative damage, and ferroptosis pathways. The combination of pevonedistat plus belinosat is safe in an adult relapsed and/or refractory AML/High-Risk MDS population with modest but notable activity in this heavily treated, high risk population. Our findings also raise the possibility that certain extremely poor prognosis AML patients may respond to a regimen combining two targeted agents that have little or no activity when administered individually.ClinicalTrials.gov ID NCT03772925, first posted 12/12/2018; CTEP Identifier 10246.© 2024. The Author(s).
Two machine learning-derived nomogram for predicting the occurrence and severity of acute graft-versus-host disease: a retrospective study based on serum biomarkersHe, Li, Fang
et alFront Genet (2024) 15, 1421980
Abstract: Acute graft-versus-host disease (aGVHD) is a common complication after allogeneic hematopoietic cell transplantation (allo-HSCT), with high morbidity and mortality. Although glucocorticoids are the standard treatment, only half of patients achieve complete remission. Thus, there is an urgent need to screen biomarkers for the diagnosis of aGVHD to assist in the identification of individuals at risk of aGVHD. This study was to construct prediction models for the occurrence and severity of aGVHD using two machine learning algorithms based on serum biochemical data.Clinical data of 120 patients with hematological diseases who received allo-HSCT were retrospectively analyzed. Seventy-six patients developed aGVHD, including 56 grade I/II and 20 grade III/IV. First, 15 serum biochemical indicators were considered as potential risk factors, and the differences in the levels of indicators between non-aGVHD and aGVHD were observed, followed by evaluation of the diagnostic property. Subsequently, to develop the prediction models for the occurrence and severity of aGVHD, LASSO and random forest (RF) analyses were performed with experimental indicators. Finally, Venn diagram analysis was utilized to obtain shared biomarkers in the two algorithms to construct the nomogram. The model performance was measured by calibration curves. Internal and external validations were performed based on risk score models and ROC curve analyses.Total 12 of 15 indicators exhibited significant differences between the aGVHD and non-aGVHD groups, with AUC values > 0.75. In machine learning analysis, eight features (LAG-3, TLR-2, PD-L1, IP-10, elafin, REG-3α, ST2, TIM3) and seven variables (LAG-3, TLR-2, PD-1, Flt_3, IL-9, elafin, TIM3) were selected to distinguish aGVHD vs. non-aGVHD as well as grade I/II vs. III/IV, respectively. Further, the corresponding nomogram models were established and calibration curves showed that prediction was in good agreement with the actual probability. Biomarker-based risk score model was constructed, which obtained AUC value >0.89 in internal and external datasets.Clinical variables screened through learning algorithm can predict the risk and severity of aGVHD. Our findings may help clinicians develop more personalized and reasonable management strategies.Copyright © 2024 He, Li, Fang, Kong, Yu and Xie.

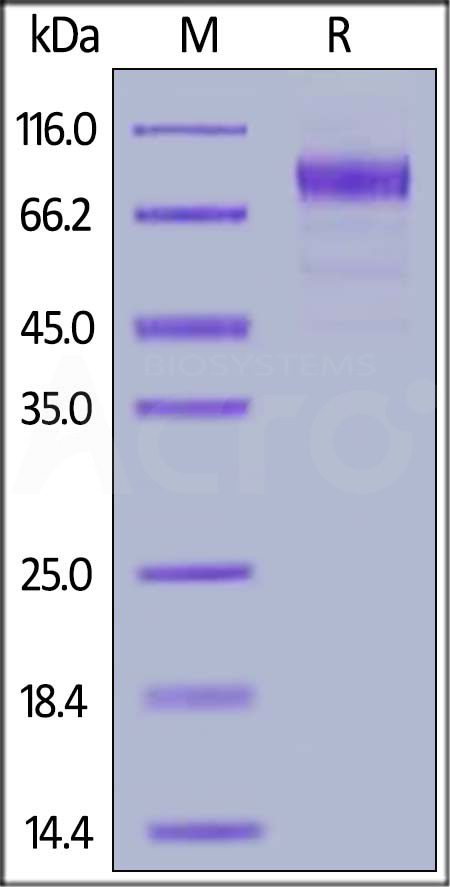
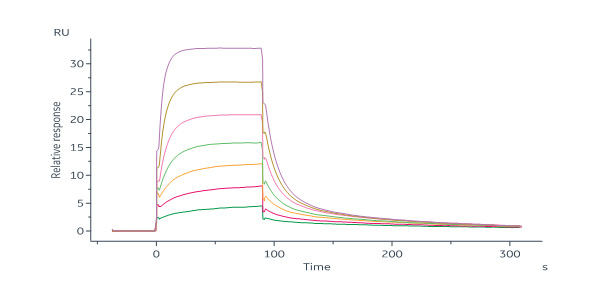























































 膜杰作
膜杰作 Star Staining
Star Staining















