Comparison of HER2 Scoring Systems in Endometrial Cancer: Toward Optimization of HER2-Directed TherapiesMenshikova, Deeb, Genega
et alAm J Surg Pathol (2025)
Abstract: Endometrial carcinomas (EC) show variable HER2 protein expression or gene amplification and may be eligible for HER2-directed therapy (trastuzumab or antibody-drug conjugates (ADCs) such as trastuzumab-deruxtecan). HER2 testing is currently recommended in advanced-stage/recurrent serous carcinomas and carcinosarcomas. However, no universally adopted reporting guidelines exist, and institutional practices vary. We aimed to analyze our experience with HER2 testing and compare gynecologic (GyC), gastric (GaC), and breast (BrC) criteria. We identified ECs with available HER2 immunohistochemistry (IHC) and fluorescence in-situ hybridization (FISH) results where applicable. HER2 IHC was reassessed using GyC, GaC, and BrC. The overall HER2-positivity rates were 31% by GyC and BrC, and 35.7% by GaC. The scoring systems significantly differed, with 69.8% concordance between GaC and GyC (P<0.001) and 99.2% concordance between BrC and GyC. Our results emphasize the importance of using the appropriate HER2 scoring criteria depending on the type of intended HER2-directed therapy as well as comprehensive yet perspicuous reporting of HER2 status to ensure optimal clinical outcomes in EC patients.Copyright © 2025 Wolters Kluwer Health, Inc. All rights reserved.
Optimized Protein-Excipient Interactions in the Martini 3 Force FieldPrass, Lindorff-Larsen, Garidel
et alJ Chem Inf Model (2025)
Abstract: The high doses of drugs required for biotherapeutics, such as monoclonal antibodies (mAbs), and the small volumes that can be administered to patients by subcutaneous injections pose challenges due to high-concentration formulations. The addition of excipients, such as arginine and glutamate, to high-concentration protein formulations can increase solubility and reduce the tendency of protein particle formation. Molecular dynamics (MD) simulations can provide microscopic insights into the mode of action of excipients in mAb formulations but require large system sizes and long time scales that are currently beyond reach at the fully atomistic level. Computationally efficient coarse-grained models such as the Martini 3 force field can tackle this challenge but require careful parametrization, testing, and validation. This study extends the popular Martini 3 force field toward realistic protein-excipient interactions of arginine and glutamate excipients, using the Fab domains of the therapeutic mAbs trastuzumab and omalizumab as model systems. A novel all-atom to coarse-grained mapping of the amino acid excipients is introduced, which explicitly captures the zwitterionic character of the backbone. The Fab-excipient interactions of arginine and glutamate are characterized concerning molecular contacts with the Fabs at the single-residue level. The Martini 3 simulations are compared with results from all-atom simulations as a reference. Our findings reveal an overestimation of Fab-excipient contacts with the default interaction parameters of Martini 3, suggesting a too strong attraction between protein residues and excipients. Therefore, we reparametrized the protein-excipient interaction parameters in Martini 3 against all-atom simulations. The excipient interactions obtained with the new Martini 3 mapping and Lennard-Jones (LJ) interaction parameters, coined Martini 3-exc, agree closely with the all-atom reference data. This work presents an improved parameter set for mAb-arginine and mAb-glutamate interactions in the Martini 3 coarse-grained force field, a key step toward large-scale coarse-grained MD simulations of high-concentration mAb formulations and the stabilizing effects of excipients.
Effects of trastuzumab emtansine on canine osteosarcoma cellsSakai, Kato, Yoshinaka
et alJ Vet Med Sci (2025)
Abstract: Human epidermal growth factor receptor 2 (HER2) is a known therapeutic target in canine osteosarcoma (OSA); however, the efficacy of anti-HER2 antibody drugs remains unclear. This study aimed to investigate the effects of the anti-HER2 antibody drugs including trastuzumab and trastuzumab emtansine (T-DM1) on canine OSA cell lines in vitro and in vivo. Four canine OSA cell lines (HMPOS, POS, OOS, and HOS) were used. Western blotting revealed HER2 protein expression in all the cell lines. Although water-soluble tetrazolium salt assays showed growth inhibitory activity of trastuzumab and T-DM1 against all the cell lines in vitro, the activity of T-DM1 was significantly stronger than that of trastuzumab. Flow cytometric analysis of the canine OSA cell line (HMPOS) revealed that T-DM1, but not trastuzumab, significantly increased the sub-G1 phase fraction in cell cycle analyses and the percentage of early and late apoptotic cells in annexin V apoptotic assays. For in vivo experiments, canine OSA cells (HMPOS) were subcutaneously injected into nude mice. Six days after inoculation, trastuzumab, T-DM1, or the vehicle control was administered intraperitoneally once per week. Survival until the tumor volume in the canine OSA-engrafted mice reached mean final tumor volume in the T-DM1 group was significantly longer in the T-DM1 group, but not in the trastuzumab group, compared to the vehicle control group. These findings indicated that T-DM1 exerts antitumor effects on canine OSA cells in vitro and in vivo, possibly by inducing apoptosis due to DM1.
IRSN-23 gene diagnosis enhances breast cancer subtype classification and predicts response to neoadjuvant chemotherapy: new validation analysesSota, Seno, Naoi
et alBreast Cancer (2025)
Abstract: This study evaluates the reproducibility of the IRSN-23 model, which classifies patients into highly chemotherapy-sensitive (Gp-R) or less-sensitive (Gp-NR) groups based on immune-related gene expression using DNA microarray analysis, and its impact on breast cancer subtype classification.Tumor tissues from 146 breast cancer patients receiving neoadjuvant chemotherapy (paclitaxel-FEC) ± trastuzumab at Osaka University Hospital (OUH) were used to classify patients into Gp-R or Gp-NR using IRSN-23. The ability to predict a pathological complete response (pCR) was assessed and the results were validated with independent public datasets (N = 1282).In the OUH dataset, the pCR rate was significantly higher in the Gp-R group than in the Gp-NR group without trastuzumab (29 versus 1%, P = 1.70E-5). In all validation sets without anti-HER2 therapy, the pCR rate in the Gp-R group was significantly higher than that in the Gp-NR group. The pooled analysis of the validation set showed higher pCR rates in the Gp-R group than in the Gp-NR group, both without (N = 1103, 40 versus 12%, P = 2.02E-26) and with (N = 304, 49 versus 35%, P = 0.017) anti-HER2 therapy. Collaboration analyses of IRSN-23 and Oncotype Dx or PAM50 could identify highly chemotherapy-sensitive groups and refine breast cancer subtype classification based on the tumor microenvironment (offensive factor-PAM50 and defensive factor-IRSN-23), and the immune subtype was correlated with a better prognosis after NAC.This study offers new validation analyses of IRSN-23 in predicting chemotherapy efficacy, showing high reproducibility. The findings indicate the clinical value of using IRSN-23 for refining breast cancer subtype classification, with implications for personalized treatment strategies and improved patient outcomes.© 2025. The Author(s).

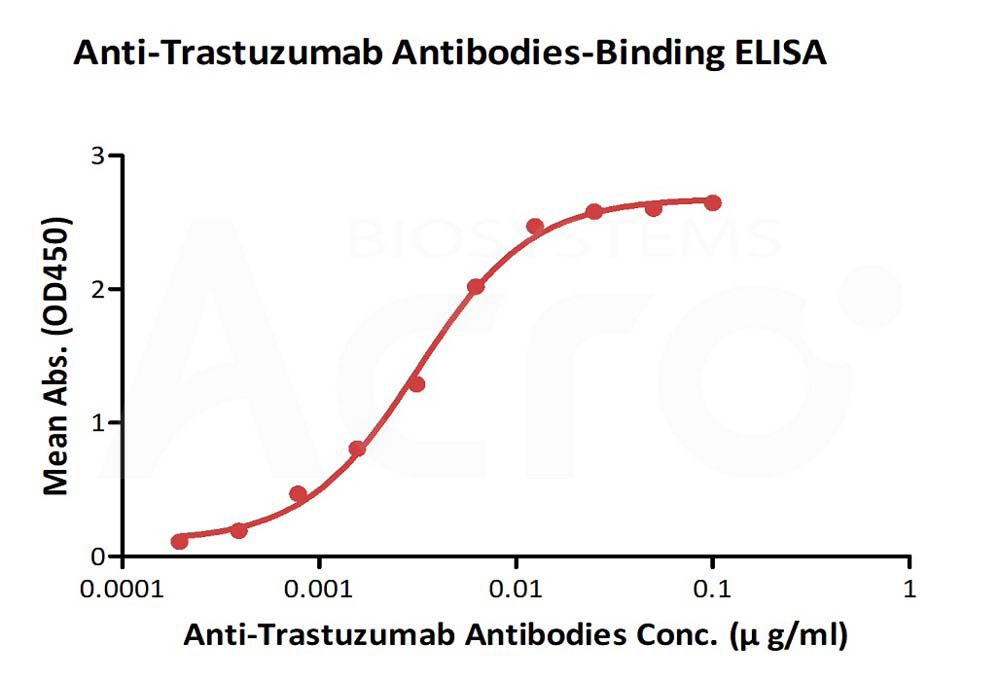
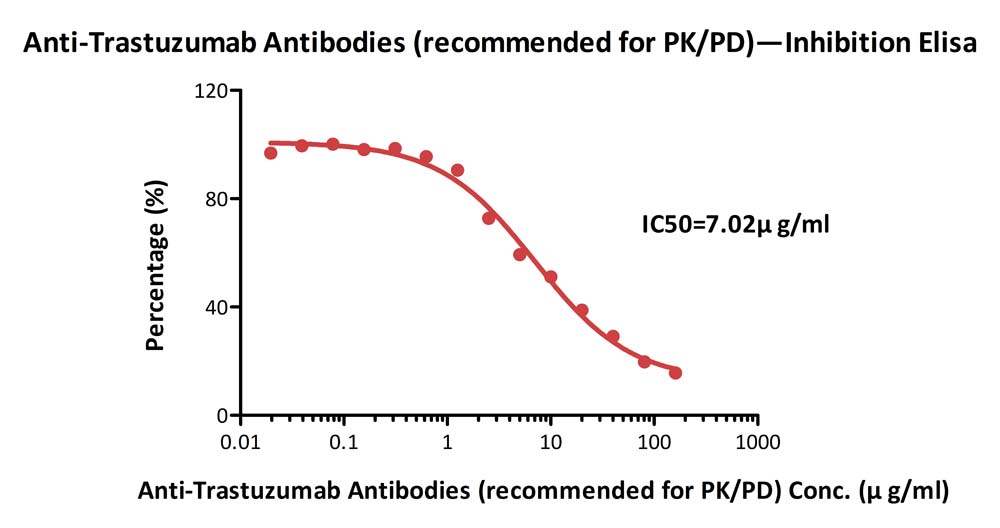
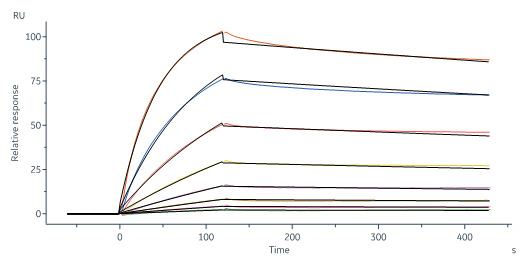
 +添加评论
+添加评论

























































 膜杰作
膜杰作 Star Staining
Star Staining















