分子别名(Synonym)
IgG1
表达区间及表达系统(Source)
Human IgG1 Fc (C103S), Flag Tag (IG1-H52C9) is expressed from human 293 cells (HEK293). It contains AA Pro 100 - Lys 330 (Accession # P01857-1(C103S)).
Predicted N-terminus: Pro 100
Request for sequence
蛋白结构(Molecular Characterization)
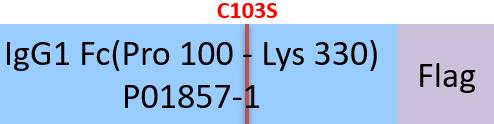
This protein carries a flag tag at the C-terminus.
The protein has a calculated MW of 27.1 kDa. The protein migrates as 28-33 kDa under reducing (R) condition (SDS-PAGE) due to glycosylation.
内毒素(Endotoxin)
Less than 1.0 EU per μg by the LAL method.
纯度(Purity)
>95% as determined by SDS-PAGE.
>95% as determined by SEC-MALS.
制剂(Formulation)
Lyophilized from 0.22 μm filtered solution in Tris with Glycine, Arginine and NaCl, pH7.5. Normally trehalose is added as protectant before lyophilization.
Contact us for customized product form or formulation.
重构方法(Reconstitution)
Please see Certificate of Analysis for specific instructions.
For best performance, we strongly recommend you to follow the reconstitution protocol provided in the CoA.
存储(Storage)
For long term storage, the product should be stored at lyophilized state at -20°C or lower.
Please avoid repeated freeze-thaw cycles.
This product is stable after storage at:
- -20°C to -70°C for 12 months in lyophilized state;
- -70°C for 3 months under sterile conditions after reconstitution.
质量管理控制体系(QMS)
电泳(SDS-PAGE)
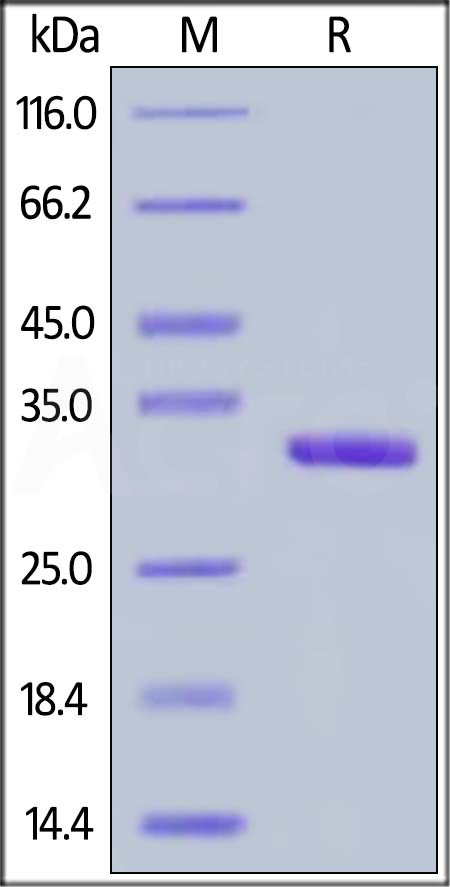
Human IgG1 Fc (C103S), Flag Tag on SDS-PAGE under reducing (R) condition. The gel was stained with Coomassie Blue. The purity of the protein is greater than 95%.
SEC-MALS
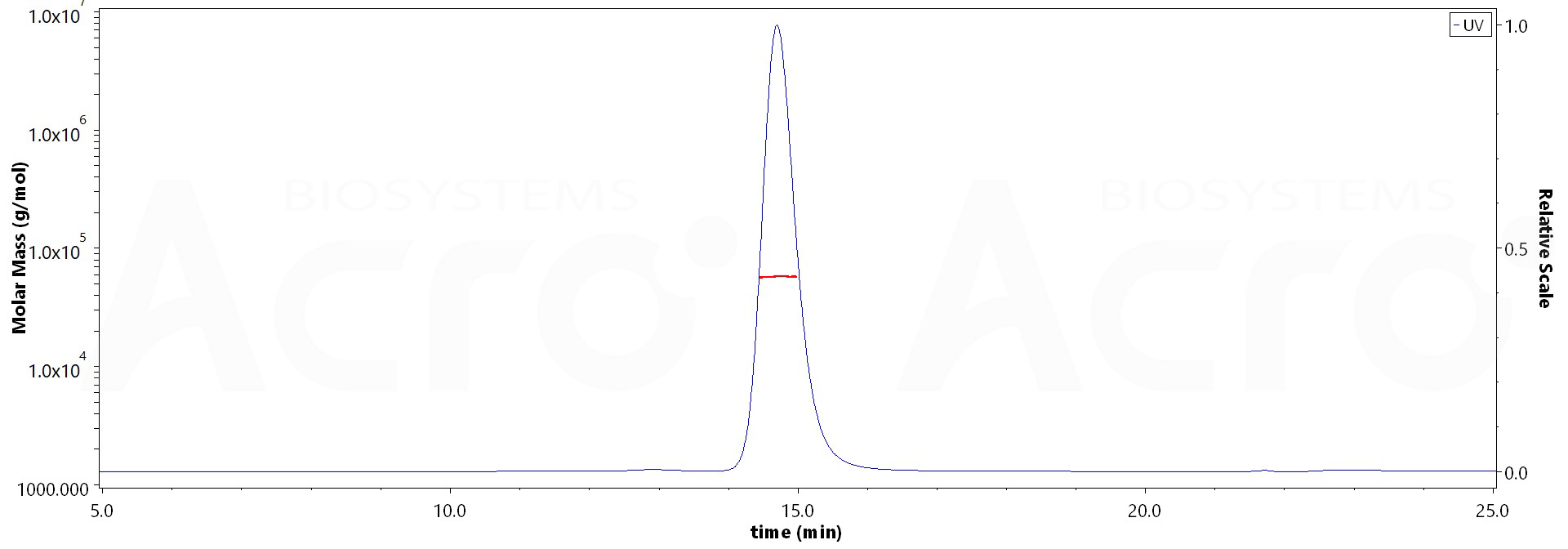
The purity of Human IgG1 Fc (C103S), Flag Tag (Cat. No. IG1-H52C9) is more than 95% and the molecular weight of this protein is around 51-63 kDa verified by SEC-MALS.
Report
背景(Background)
Crystallizable fragments composed of the carboxy-terminal halves of both IMMUNOGLOBULIN HEAVY CHAINS linked to each other by disulfide bonds. Fc fragments contain the carboxy-terminal parts of the heavy chain constant regions that are responsible for the effector functions of an immunoglobulin (COMPLEMENT fixation, binding to the cell membrane via FC RECEPTORS, and placental transport). IgG1 Fc was reported has a novel role as a potential anti-inflammatory drug for treatment of human autoimmune diseases.























































 膜杰作
膜杰作 Star Staining
Star Staining















