分子别名(Synonym)
RP1-261G23.1,MGC70609,MVCD1,VEGFA,VPF
表达区间及表达系统(Source)
Human VEGF165, premium grade (VE5-H4210) is expressed from human 293 cells (HEK293). It contains AA Ala 27 - Arg 191 (Accession # P15692-4).
Predicted N-terminus: Ala 27
It is produced under our rigorous quality control system that incorporates a comprehensive set of tests including sterility and endotoxin tests. Product performance is carefully validated and tested for compatibility for cell culture use or any other applications in the early preclinical stage.
GMP-VE5H23 is the GMP version of this VE5-H4210. These two proteins display indistinguishable performance profiles, thereby ensuring a seamless transition for end users from early preclinical stag to later clinical phases.
Request for sequence
蛋白结构(Molecular Characterization)

This protein carries no "tag".
The protein has a calculated MW of 19.2 kDa. The protein migrates as 24 kDa±3 kDa when calibrated against Star Ribbon Pre-stained Protein Marker under reducing (R) condition (SDS-PAGE) due to glycosylation.
内毒素(Endotoxin)
Less than 0.01 EU per μg by the LAL method.
宿主蛋白残留(Host Cell Protein)
<0.5 ng/µg of protein tested by ELISA.
宿主核酸残留(Host Cell DNA)
<0.02 ng/μg of protein tested by qPCR.
无菌(Sterility)
Negative
支原体(Mycoplasma)
Negative.
纯度(Purity)
>95% as determined by SDS-PAGE.
>95% as determined by SEC-MALS.
制剂(Formulation)
Lyophilized from 0.22 μm filtered solution in PBS, pH7.4 with trehalose as protectant.
Contact us for customized product form or formulation.
重构方法(Reconstitution)
Please see Certificate of Analysis for specific instructions.
For best performance, we strongly recommend you to follow the reconstitution protocol provided in the CoA.
存储(Storage)
For long term storage, the product should be stored at lyophilized state at -20°C or lower.
Please avoid repeated freeze-thaw cycles.
This product is stable after storage at:
- -20°C to -70°C for 24 months in lyophilized state;
- -70°C for 3 months under sterile conditions after reconstitution.
质量管理控制体系(QMS)
电泳(SDS-PAGE)
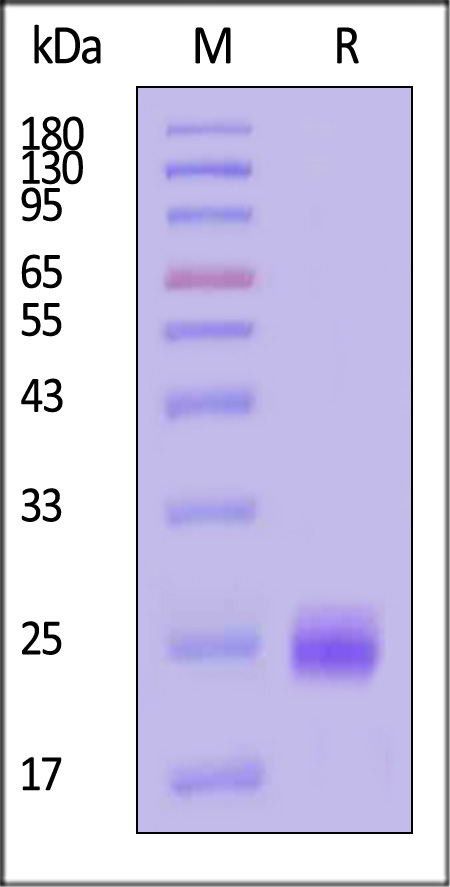
Human VEGF165, premium grade on SDS-PAGE under reducing (R) condition. The gel was stained with Coomassie Blue. The purity of the protein is greater than 95% (With Star Ribbon Pre-stained Protein Marker).
SEC-MALS

The purity of Human VEGF165, premium grade (Cat. No. VE5-H4210) is more than 95% and the molecular weight of this protein is around 42-60 kDa verified by SEC-MALS.
Report
活性(Bioactivity)-CELL BASE

Human VEGF165, premium grade (Cat. No. VE5-H4210) stimulates proliferation of 293F-NFAT/Luc-VEGFR2-3-7 cells. The specific activity of Human VEGF165, premium grade is>8.00 x 10^5 IU/mg, which is calibrated against human vascular endothelial growth factor 165 WHO International Standard (NIBSC code: 02/286) (QC tested).
Protocol

Human VEGF165, premium grade (Cat. No. VE5-H4210) stimulates proliferation of human umbilical vein endothelial cells (HUVEC). The ED50 for this effect is 4.216-9.281 ng/mL (Routinely tested).
Protocol

Inhibition assay shows that the proliferation effect of Human VEGF165, premium grade (Cat. No. VE5-H4210) is inhibited by increasing concentration of anti-VEGF mAb (Avastin). The concentration of VEGF165 used is 20 ng/mL. The ED50 is 0.065-0.229 μg/mL (Routinely tested).
Protocol

Response to human VEGF165 protein (Fold).
The VEGFR2 (Luc) HEK293 Reporter Cell was stimulated with serial dilutions of human VEGF165 protein (AcroBiosystems, Cat. No. VE5-H4210). The max induction fold was approximately 70 (Routinely tested).
Protocol
活性(Bioactivity)-ELISA

Immobilized Human VEGF165, premium grade (Cat. No. VE5-H4210) at 0.1 μg/mL (100 μL/well) can bind Human VEGF R1 Protein, His Tag (Cat. No. VE1-H52H9) with a linear range of 4-31 ng/mL (QC tested).
Protocol

Immobilized Human VEGF165, premium grade (Cat. No. VE5-H4210) at 2 μg/mL (100 μL/well) can bind Biotinylated Human VEGF R2, Avitag,His Tag (Cat. No. KDR-H82E5) with a linear range of 10-156 ng/mL (Routinely tested).
Protocol

Immobilized Human VEGF165, premium grade (Cat. No. VE5-H4210) at 2 μg/mL (100 μL/well) can bind pre-mixed increasing concentrations of Bevacizumab and 0.5 μg/mL (100 μL/well) Biotinylated Human VEGF R2, Avitag,His Tag (Cat. No. KDR-H82E5) with a half maximal inhibitory concentration (IC50) of 0.70 μg/mL (Routinely tested).
Protocol
活性(Bioactivity)-SPR
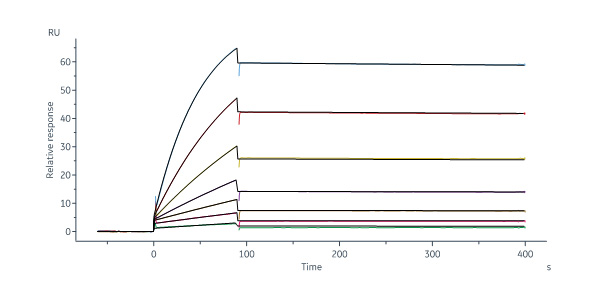
Anti-VEGFA Antibody, Human IgG1 captured on Protein A Chip can bind Human VEGF165, premium grade (Cat. No. VE5-H4210) with an affinity constant of 0.103 nM as determined in a SPR assay (Biacore 8K) (Routinely tested).
Protocol
活性(Bioactivity)-BLI

Loaded bind Anti-VEGFA Antibody, Human IgG1 on AHC2 Biosensor, can bind Human VEGF165, premium grade (Cat. No. VE5-H4210) with an affinity constant of 53.8 pM as determined in BLI assay (ForteBio Octet Red96e) (Routinely tested).
Protocol

Loaded bind Human NRP1, Fc Tag (Cat. No. NR1-H5252) on Protein A Biosensor, can bind Human VEGF165, premium grade (Cat. No. VE5-H4210) with an affinity constant of 17.3 nM as determined in BLI assay (ForteBio Octet Red96e) (Routinely tested).
Protocol
稳定性(Stability)
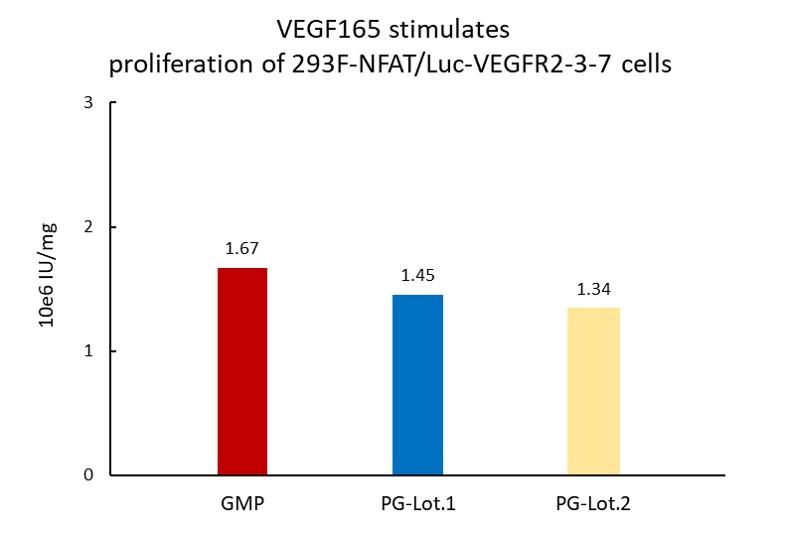
The Cell based assay shows batch-to-batch consistency between Acro's GMP and PG VEGF165.
 +添加评论
+添加评论
- 192XXXXXXX8
- 我们购买此蛋白主要是用于VEGF 促进HUVEC细胞增殖实验,结果显示在80ng/mL时能显著促进细胞增殖,且重复性好,进一步促进了我们的实验进度,感谢ACRO。

 >
>- 2024-8-13

- 185XXXXXXX1
- 之前购买其他进口品牌VEGF165进行亲和力测定,结果一直不稳定,后来尝试用了一下ACRO的VEGF165,发现比进口品牌稳定的多,ACRO质量确实值得信任。
 >
>- 2022-6-20
背景(Background)
VEGF165 is the most abundant splice variant of VEGF-A. VEGF165 is produced by a number of cells including endothelial cells, macrophages and T cells. VEGF165 is involved in angiogenesis, vascular endothelial cell survival, growth, migration and vascular permeability. VEGF gene expression is induced by hypoxia, inflammatory cytokines and oncogenes. VEGF165 binds to heparan sulfate and is retained on the cell surface and in the extracellular matrix. VEGF165 binds to the receptor tyrosine kinases, VEGFR1 and VEGFR2. VEGF165 is the only splice variant that binds to co-receptors NRP-1 and NRP-2 that function to enhance VEGFR2 signaling. Binding of VEGF165 to VEGFR1 and VEGFR2 leads to activation of the PI3K/AKT, p38 MAPK, FAK and paxillin. VEGF plays a key role in tumor angiogenesis in many cancers.























































 膜杰作
膜杰作 Star Staining
Star Staining
















