分子别名(Synonym)
CD19,B4,CVID3,MGC12802
表达区间及表达系统(Source)
Biotinylated Human CD19 (20-291), His,Avitag, premium grade (CD9-H82E9) is expressed from human 293 cells (HEK293). It contains AA Pro 20 - Lys 291 (Accession # P15391-1).
Predicted N-terminus: Pro 20
It is produced under our rigorous quality control system that incorporates a comprehensive set of tests including sterility and endotoxin tests. Product performance is carefully validated and tested for compatibility for cell culture use or any other applications in the early preclinical stage. When ready to transition into later clinical phases, we also offer a custom GMP protein service that tailors to your needs. We will work with you to customize and develop a GMP-grade product in accordance with your requests that also meets the requirements for raw and ancillary materials use in cell manufacturing of cell-based therapies.
Request for sequence
蛋白结构(Molecular Characterization)

This protein carries a polyhistidine tag at the C-terminus, followed by an Avi tag (Avitag™).
The protein has a calculated MW of 33.7 kDa. The protein migrates as 50-65 kDa when calibrated against Star Ribbon Pre-stained Protein Marker under reducing (R) condition (SDS-PAGE) due to glycosylation.
标记(Labeling)
Biotinylation of this product is performed using Avitag™ technology. Briefly, the single lysine residue in the Avitag is enzymatically labeled with biotin.
蛋白标记度(Protein Ratio)
Passed as determined by the HABA assay / binding ELISA.
内毒素(Endotoxin)
Less than 0.01 EU per μg by the LAL method.
无菌(Sterility)
Negative
支原体(Mycoplasma)
Negative.
纯度(Purity)
>90% as determined by SDS-PAGE.
制剂(Formulation)
Lyophilized from 0.22 μm filtered solution in PBS, pH7.4 with trehalose as protectant.
Contact us for customized product form or formulation.
重构方法(Reconstitution)
Please see Certificate of Analysis for specific instructions.
For best performance, we strongly recommend you to follow the reconstitution protocol provided in the CoA.
存储(Storage)
For long term storage, the product should be stored at lyophilized state at -20°C or lower.
Please avoid repeated freeze-thaw cycles.
This product is stable after storage at:
- -20°C to -70°C for 12 months in lyophilized state;
- -70°C for 3 months under sterile conditions after reconstitution.
质量管理控制体系(QMS)
电泳(SDS-PAGE)
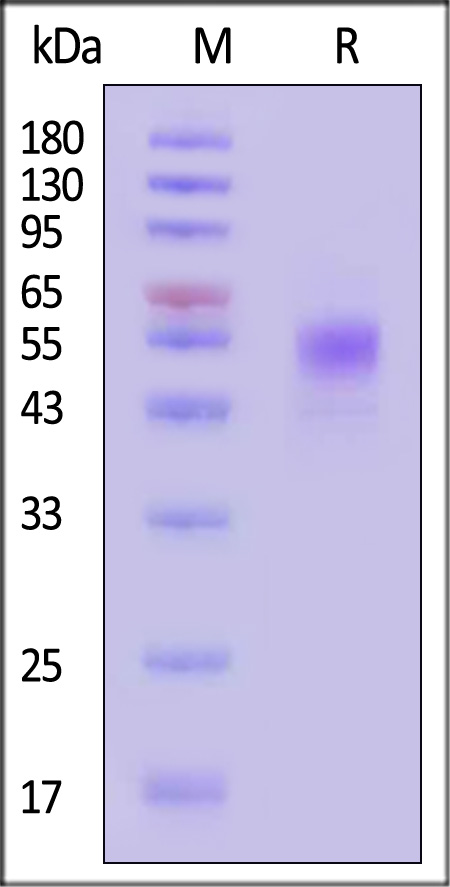
Biotinylated Human CD19 (20-291), His,Avitag, premium grade on SDS-PAGE under reducing (R) condition. The gel was stained with Coomassie Blue. The purity of the protein is greater than 90% (With Star Ribbon Pre-stained Protein Marker).
活性(Bioactivity)-FACS
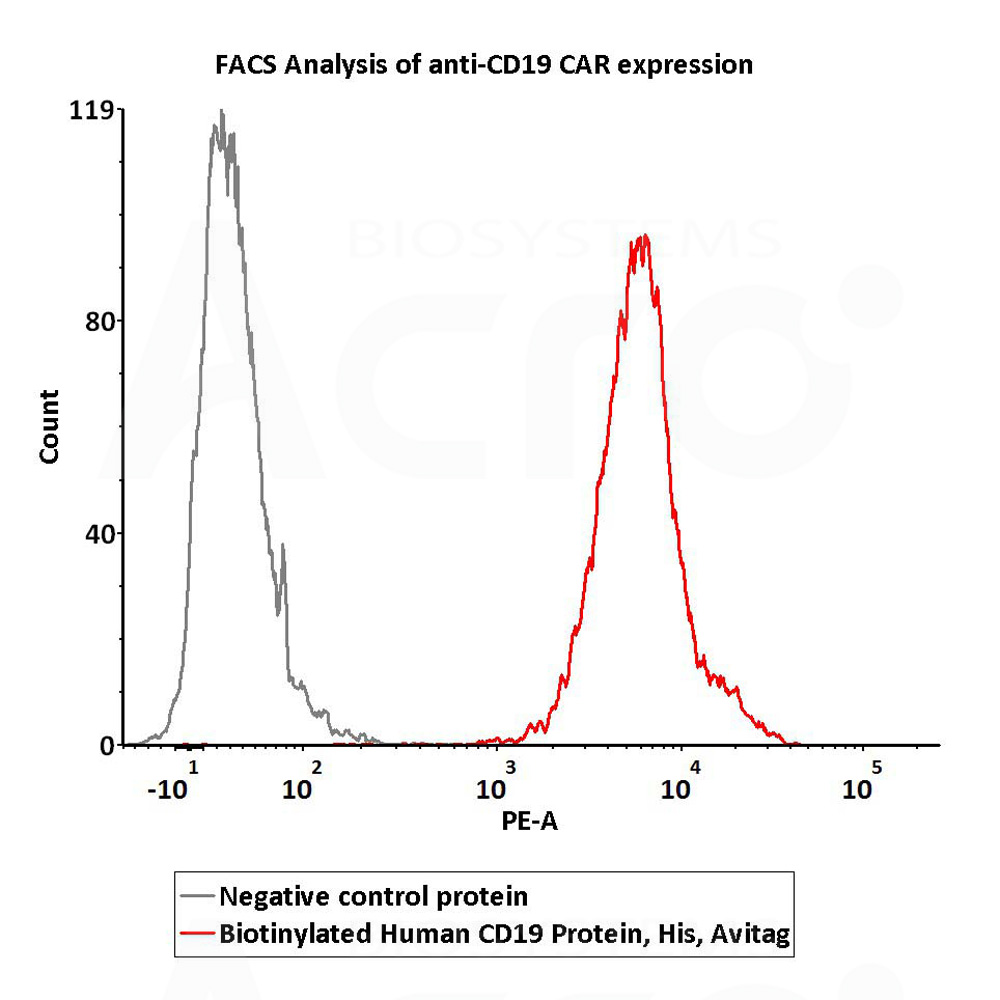
2e5 of anti-CD19 CAR-293 cells were stained with 100 μL of 1 μg/mL of Biotinylated Human CD19 (20-291), His,Avitag, premium grade (Cat. No. CD9-H82E9) and negative control protein respectively, washed and then followed by PE-SA and analyzed with FACS (QC tested).
Protocol
活性(Bioactivity)-ELISA
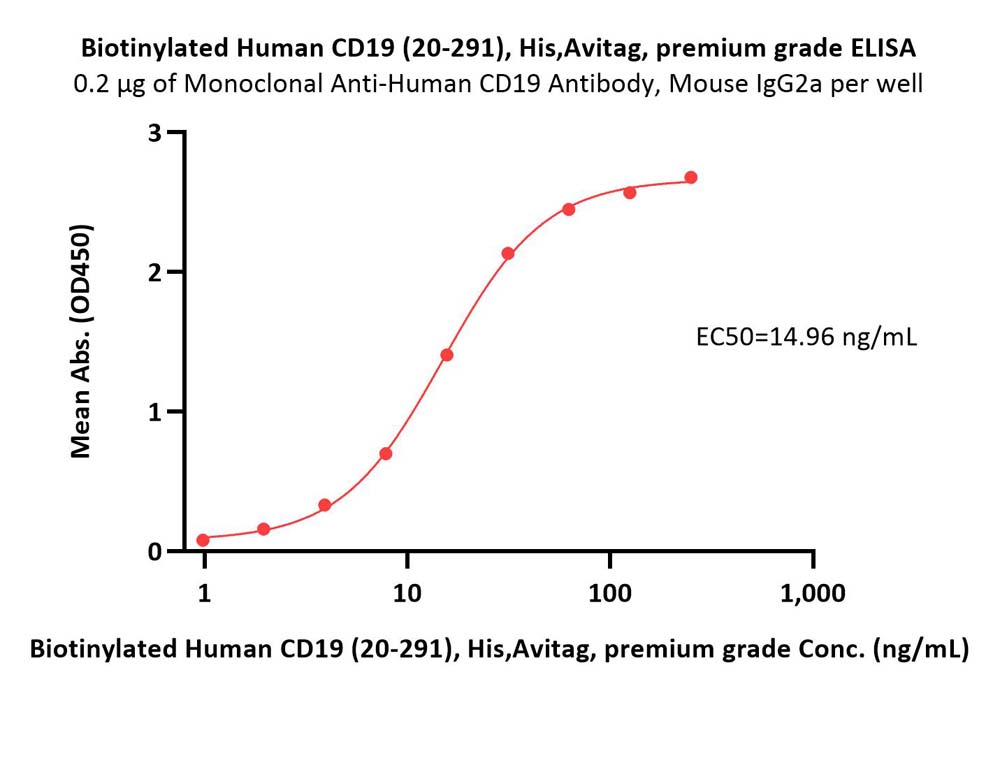
Immobilized Monoclonal Anti-Human CD19 Antibody, Mouse IgG2a at 2 μg/mL (100 μL/well) can bind Biotinylated Human CD19 (20-291), His,Avitag, premium grade (Cat. No. CD9-H82E9) with a linear range of 1-31 ng/mL (Routinely tested).
Protocol
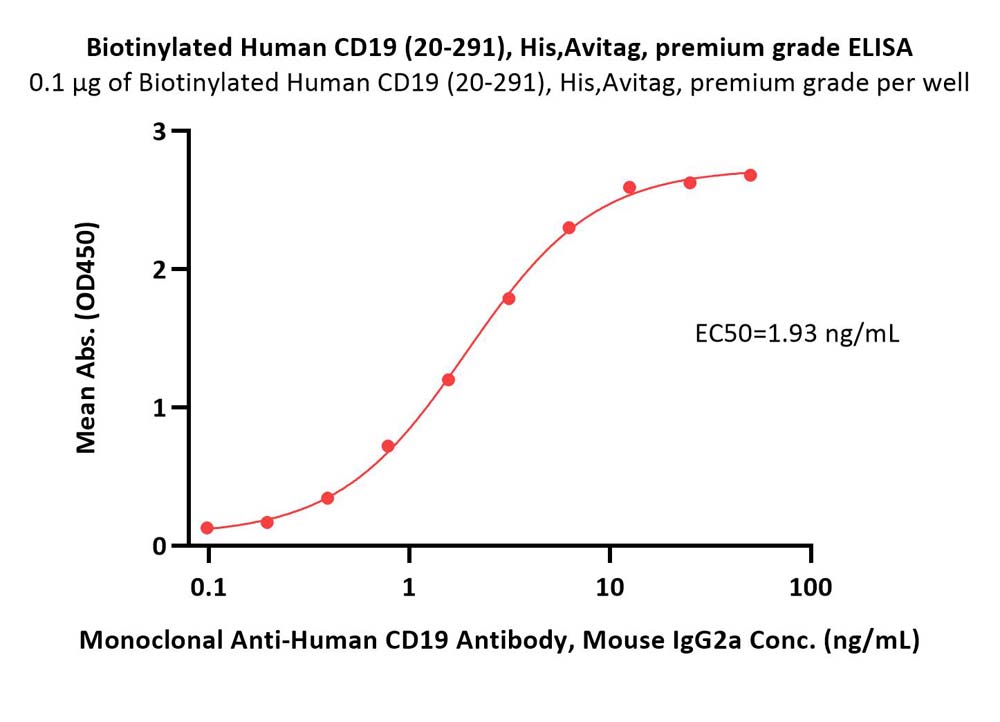
Immobilized Biotinylated Human CD19 (20-291), His,Avitag, premium grade (Cat. No. CD9-H82E9) at 1 μg/mL (100 μL/well) on Streptavidin (Cat. No. STN-N5116) precoated (0.5 μg/well) plate, can bind Monoclonal Anti-Human CD19 Antibody, Mouse IgG2a with a linear range of 0.1-6 ng/mL (Routinely tested).
Protocol
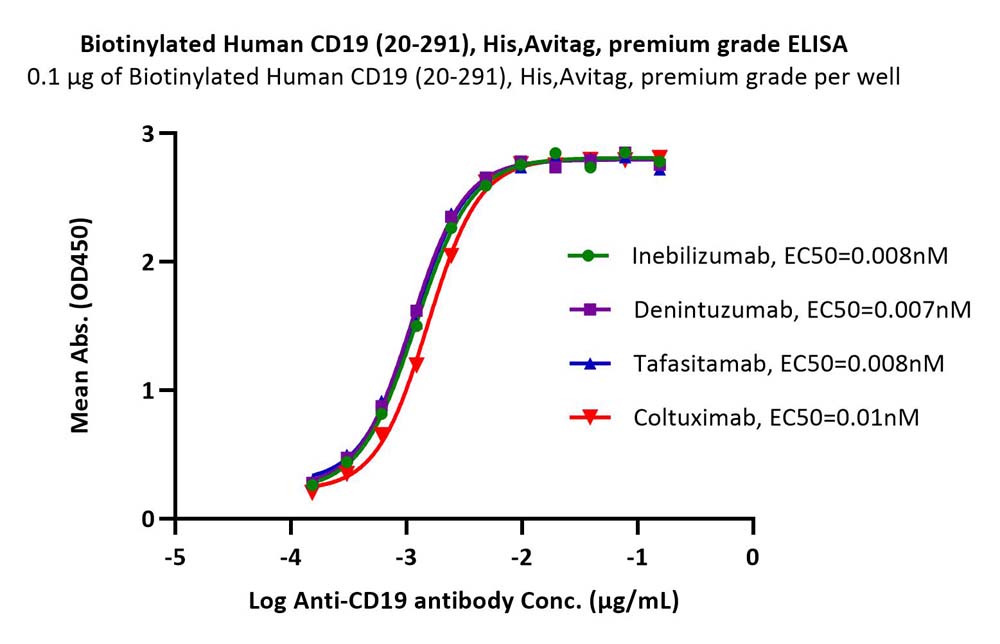
Immobilized Biotinylated Human CD19 (20-291), His,Avitag, premium grade (Cat. No. CD9-H82E9) at 1 μg/mL (100 μL/well) on streptavidin (Cat. No. STN-N5116) precoated (0.5 μg/well) plate can bind different Anti-CD19 antibodies with high affinity (Routinely tested).
Protocol
活性(Bioactivity)-SPR
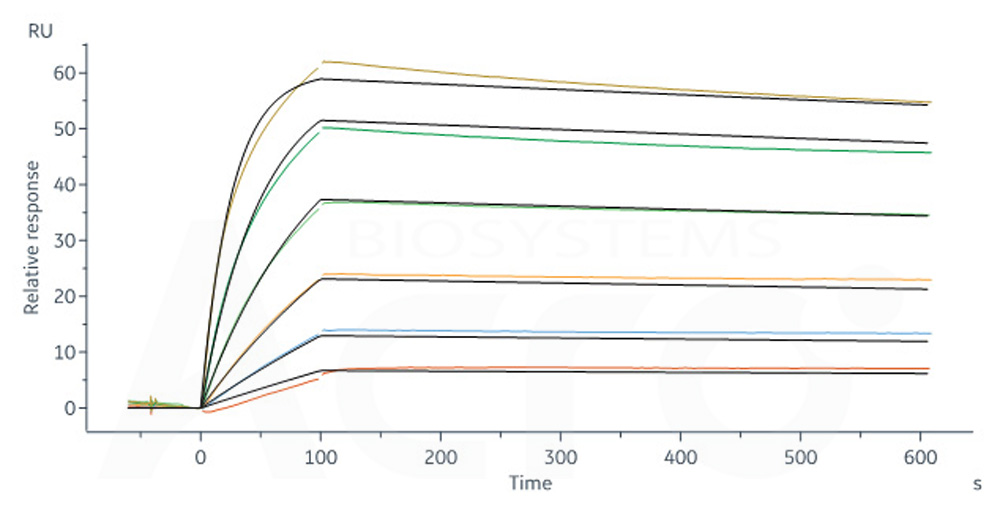
Biotinylated Human CD19 (20-291), His,Avitag, premium grade (Cat. No. CD9-H82E9) captured on Biotin CAP - Series S sensor Chip can bind FMC63 MAb (mouse lgG2a) with an affinity constant of 0.255 nM as determined in a SPR assay (Biacore 8K) (QC tested).
Protocol
 +添加评论
+添加评论
- 508XXXXXXX
- We ordered this material for screening of clones via cell sorting. This arrived promptly and was excellent quality. The material bound very well and we ordered more material and of a larger size. We also were able to aliquot the sample using diH20 and stored it long term at -80. The material stored at -80 was kept for several months, and we thawed it out and were able to use it with no issues. We will definitely be ordering more!
- 2022-7-29
背景(Background)
B-lymphocyte antigen CD19 is also known as CD19 (Cluster of Differentiation 19), is a single-pass type I membrane protein which contains two Ig-like C2-type (immunoglobulin-like) domains. CD19 is expressed on follicular dendritic cells and B cells. In fact, it is present on B cells from earliest recognizable B-lineage cells during development to B-cell blasts but is lost on maturation to plasma cells. It primarily acts as a B cell co-receptor in conjunction with CD21 and CD81. Upon activation, the cytoplasmic tail of CD19 becomes phosphorylated, which leads to binding by Src-family kinases and recruitment of PI-3 kinase. As on T cells, several surface molecules form the antigen receptor and form a complex on B lymphocytes. The (almost) B cell-specific CD19 phosphoglycoprotein is one of these molecules. The others are CD21 and CD81. These surface immunoglobulin (sIg)-associated molecules facilitate signal transduction. On living B cells, anti-immunoglobulin antibody mimicking exogenous antigen causes CD19 to bind to sIg and internalize with it. The reverse process has not been demonstrated, suggesting that formation of this receptor complex is antigen-induced. This molecular association has been confirmed by chemical studies. Mutations in CD19 are associated with severe immunodeficiency syndromes characterized by diminished antibody production. CD19 has been shown to interact with: CD81, CD82, Complement receptor 2, and VAV2.























































 膜杰作
膜杰作 Star Staining
Star Staining
















