SARS CoV-2 spike adopts distinct conformational ensembles in situGramm, Braet, Srinivasu
et albioRxiv (2025)
Abstract: Engineered recombinant Spike (S) has been invaluable for determining S structure and dynamics and is the basis for the design of most prevalent vaccines. While these vaccines have been highly efficacious for short-term protection from infection, protection waned with the emergence of variants (alpha through omicron). Here we report differences in conformational dynamics between native, membrane-embedded full-length S and recombinant S. Our virus-like particle (VLP) model mimics the native SARS CoV-2 virion by displaying S assembled with auxiliary E, M, and N proteins in a native membrane environment that captures the entirety of quaternary interactions mediated by S. Display of S on VLP obviates the requirement for stabilizing modifications that have been engineered into recombinant S for enhanced expression and solubility. Amide hydrogen/deuterium exchange mass spectrometry (HDXMS) reveals altered interprotomer contacts in VLP S trimers attributable to the presence of auxiliary proteins, membrane anchoring, and lack of engineered modifications. Our results reveal decreased dynamics in the S2 subunit and at sites spanning interprotomer contacts in VLP S with minimal differences in the N-terminal domain (NTD) and receptor binding domain (RBD). This carries implications for display of epitopes beyond NTD and RBD. In summary, despite affording efficient structural characterization, recombinant S distorts the intrinsic conformational ensemble of native S displayed on the virus surface.
Bispecific antibodies targeting the N-terminal and receptor binding domains potently neutralize SARS-CoV-2 variants of concernRubio, Baharani, Dadonaite
et alSci Transl Med (2025) 17 (788), eadq5720
Abstract: The ongoing emergence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants of concern (VOCs) that reduce the effectiveness of antibody therapeutics necessitates development of next-generation antibody modalities that are resilient to viral evolution. Here, we characterized amino-terminal domain (NTD)- and receptor binding domain (RBD)-specific monoclonal antibodies previously isolated from coronavirus disease 2019 (COVID-19) convalescent donors for their activity against emergent SARS-CoV-2 VOCs. Among these, the NTD-specific antibody C1596 displayed the greatest breadth of binding to VOCs, with cryo-electron microscopy structural analysis revealing recognition of a distinct NTD epitope outside of the site i antigenic supersite. Given C1596's favorable binding profile, we designed a series of bispecific antibodies (bsAbs), termed CoV2-biRNs, that featured both NTD and RBD specificities. Two of the C1596-inclusive bsAbs, CoV2-biRN5 and CoV2-biRN7, retained potent in vitro neutralization activity against all Omicron variants tested, including XBB.1.5, BA.2.86, and JN.1, contrasting the diminished potency of parental antibodies delivered as monotherapies or as a cocktail. Furthermore, prophylactic delivery of CoV2-biRN5 reduced the viral load within the lungs of K18-hACE2 mice after challenge with SARS-CoV-2 XBB.1.5. In conclusion, NTD-RBD bsAbs offer promising potential for the design of resilient, next-generation antibody therapeutics against SARS-CoV-2 VOCs.
Exploring the effects of N234 and N343 linked glycans to SARS CoV 2 spike protein pocket accessibility using Gaussian accelerated molecular dynamics simulationsCheng, Lim, Fortuna
et alSci Rep (2025) 15 (1), 7052
Abstract: The N234 and N343-linked glycans of the SARS-CoV 2 spike protein are known to stabilize the up-conformation of its receptor-binding domains (RBDs), enabling human angiotensin enzyme 2 (hACE2) receptor binding. However, the effect of spike-hACE2 binding on these important glycans remains poorly understood, and these changes could have implications in the development of drugs that inhibit viral entry. In this study, Gaussian accelerated molecular dynamics (GaMD) simulations of the hACE2-free and hACE2-bound spike protein are performed. Biophysical analyses were focused on the accessibility of three previously suggested druggable pockets underneath the three RBD subunits. A shielding effect by N234-linked glycans on the components of their adjacent pockets was observed. Although deshielding of central scaffold residues was observed in the hACE2-bound state, pocket A's accessibility was reduced due to an increase in NTDB-RBDB contacts, restricting entry into the pocket. For pocket B, changes in N234C and N343C expose the central scaffold residues in the bound state, increasing accessibility. In Pocket C, increased shielding due to N234A was found in the bound state, reducing accessibility. Despite these changes, the pockets remain accessible to ligands in both states and are still valid targets for drug development studies.© 2025. The Author(s).
NIEAs elicited by wild-type SARS-CoV-2 primary infection fail to enhance the infectivity of Omicron variantsGui, Wang, Liu
et alVirol J (2025) 22 (1), 45
Abstract: SARS-CoV-2 infection widely induces antibody response targeting diverse viral proteins, including typical representative N-terminal domain (NTD), receptor-binding domain (RBD), and S2 subunit of spike. A lot of NTD-, RBD-, and S2-specific monoclonal antibodies (mAbs) have been isolated from COVID-19 convalescents, some of which displaying potent activities to inhibit viral infection. However, a small portion of NTD-specific mAbs elicited by wild-type (WT) SARS-CoV-2 primary infection could facilitate the virus entry into target cells in vitro, so called NTD-targeting infection-enhancing antibodies (NIEAs). To date, SARS-CoV-2 has evolved to massive variants carrying various NTD mutations, especially recent Omicron BA.2.86 and JN.1. In this study, we investigated whether these WT-NIEAs could still enhance the infectivity of emerging Omicron variants. Nine novel WT-NIEAs with diverse germline gene usage were identified from 3 individuals, effectively enlarging available antibody panel of NIEAs. Bivalent binding of NIEAs to inter-spike contributed to their infection-enhancing activities. WT-NIEAs could enhance the infectivity of SARS-CoV-2 variants emerged before Omicron, but ineffective to Omicron variants including BA.2.86 and JN.1, which was because of their changed antigenicity of NTDs. Overall, these data clearly demonstrated the cross-reactivity of these pre-existed WT-NIEAs to a series of SARS-CoV-2 variants, helping to evaluate the risk of enhanced infection of emerging variants in future.© 2025. The Author(s).

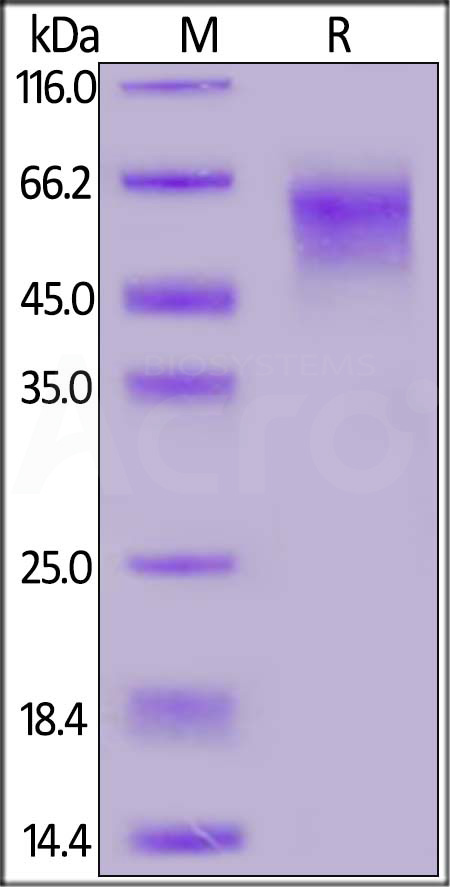

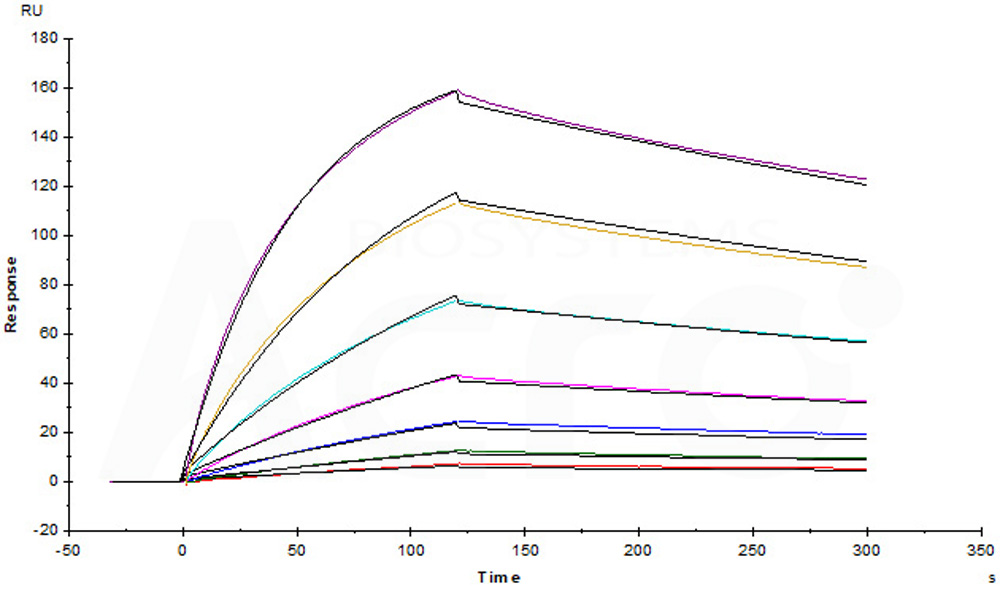
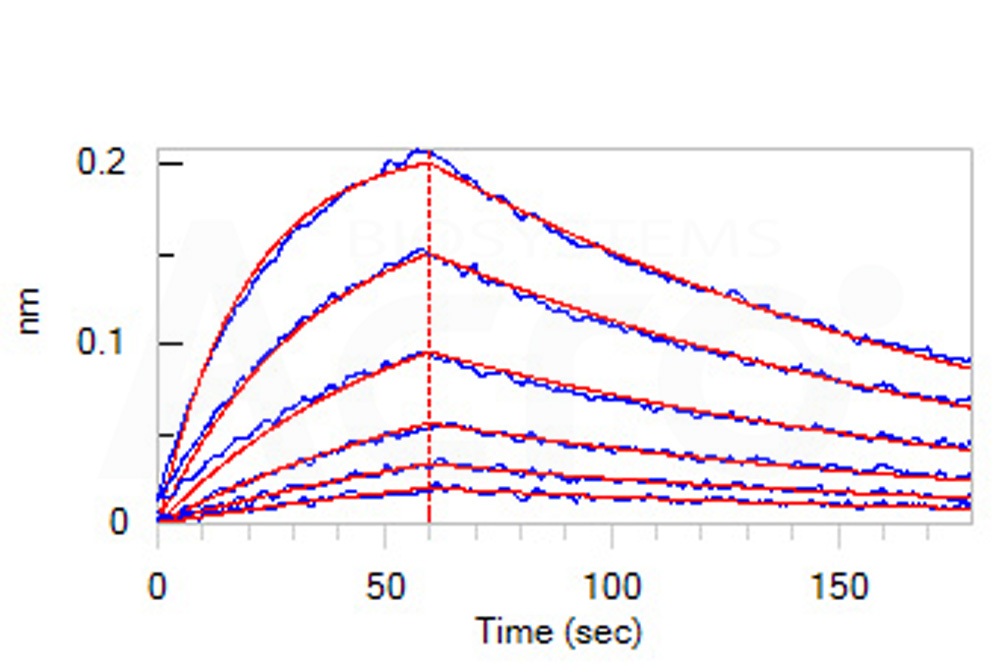
 +添加评论
+添加评论






















































 膜杰作
膜杰作 Star Staining
Star Staining
















