分子别名(Synonym)
IL33,DV27,C9ORF26,IL1F11,NFHEV,DKFZp586H0523,DVS27,NFEHEV,RP11-575C20.2
表达区间及表达系统(Source)
Biotinylated Human IL-33, His,Avitag (IL3-H82H5) is expressed from human 293 cells (HEK293). It contains AA His 109 - Thr 270 (Accession # O95760-1).
Predicted N-terminus: His
Request for sequence
蛋白结构(Molecular Characterization)

This protein carries a polyhistidine tag at the N-terminus, followed by an Avi tag (Avitag™).
The protein has a calculated MW of 21.9 kDa. The protein migrates as 28-33 kDa under reducing (R) condition (SDS-PAGE) due to glycosylation.
标记(Labeling)
Biotinylation of this product is performed using Avitag™ technology. Briefly, the single lysine residue in the Avitag is enzymatically labeled with biotin.
蛋白标记度(Protein Ratio)
Passed as determined by the HABA assay / binding ELISA.
内毒素(Endotoxin)
Less than 1.0 EU per μg by the LAL method.
纯度(Purity)
>90% as determined by SDS-PAGE.
>90% as determined by SEC-MALS.
制剂(Formulation)
Lyophilized from 0.22 μm filtered solution in PBS, pH7.4 with trehalose as protectant.
Contact us for customized product form or formulation.
重构方法(Reconstitution)
Please see Certificate of Analysis for specific instructions.
For best performance, we strongly recommend you to follow the reconstitution protocol provided in the CoA.
存储(Storage)
For long term storage, the product should be stored at lyophilized state at -20°C or lower.
Please avoid repeated freeze-thaw cycles.
This product is stable after storage at:
- -20°C to -70°C for 12 months in lyophilized state;
- -70°C for 3 months under sterile conditions after reconstitution.
质量管理控制体系(QMS)
电泳(SDS-PAGE)

Biotinylated Human IL-33, His,Avitag on SDS-PAGE under reducing (R) condition. The gel was stained with Coomassie Blue. The purity of the protein is greater than 90%.
SEC-MALS

The purity of Biotinylated Human IL-33, His,Avitag (Cat. No. IL3-H82H5) is more than 90% and the molecular weight of this protein is around 25-40 kDa verified by SEC-MALS.
Report
活性(Bioactivity)-ELISA
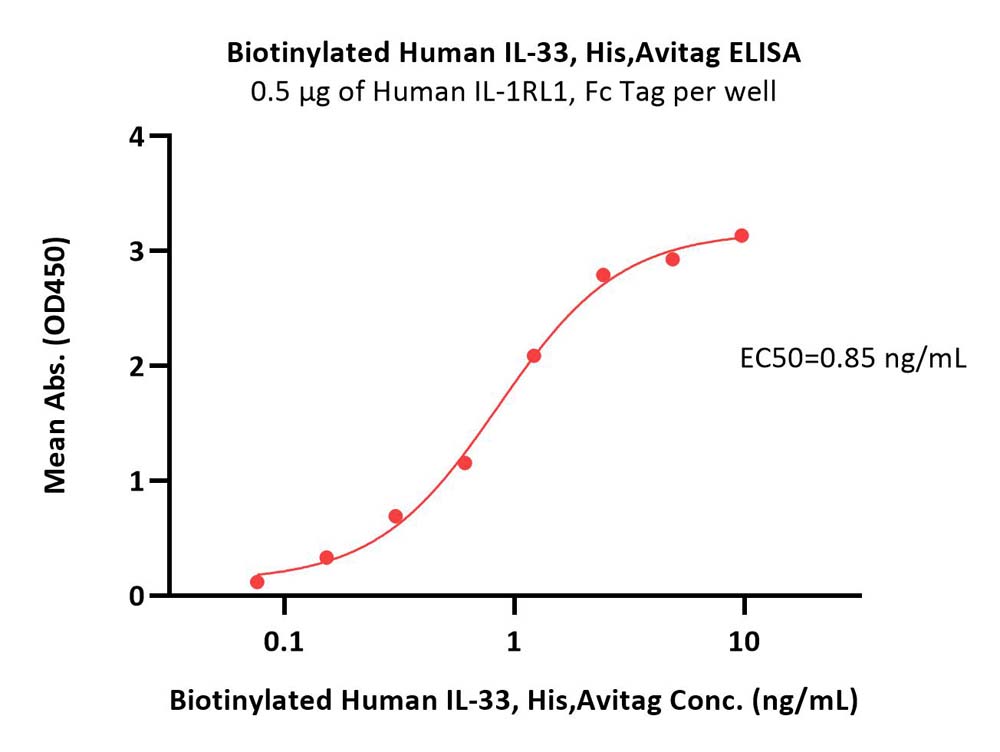
Immobilized Human IL-1RL1, Fc Tag (Cat. No. IL1-H5250) at 5 μg/mL (100 μL/well) can bind Biotinylated Human IL-33, His,Avitag (Cat. No. IL3-H82H5) with a linear range of 0.1-2 ng/mL (QC tested).
Protocol
活性(Bioactivity)-BLI

Loaded Human IL-1RL1, Fc Tag (Cat. No. IL1-H5250) on Protein A Biosensor, can bind with Biotinylated Human IL-33, His,Avitag (Cat. No. IL3-H82H5) an affinity constant of 1.81 nM as determined in BLI assay (ForteBio Octet Red96e) (Routinely tested).
Protocol
 +添加评论
+添加评论
- 176XXXXXXX2
- 用来检测细胞中和细胞分泌的S蛋白含量,细胞培液是直接加到试剂盒提供的酶标板中,贴壁的细胞是加入细胞裂解液,4度裂解一段时间反复吹打后加入酶标板中,均可以检测出S蛋白含量
- 2021-10-22
背景(Background)
Interleukin 33 (IL33) is known as C9orf26, DKFZp586H0523, DVS27, NF-HEV, NFEHEV, RP11-575C20.2,and is a cytokine belonging to the IL-1 superfamily. IL-33 induces helper T cells, mast cells, eosinophils and basophils to produce type 2 cytokines. IL-33 mediates its biological effects by interacting with the receptors ST2 (aka IL1RL1) and IL-1 Receptor Accessory Protein (IL1RAP), activating intracellular molecules in the NF-κB and MAP kinase signaling pathways that drive production of type 2 cytokines (e.g. IL-5 and IL-13) from polarized Th2 cells. In vivo, IL-33 induces the expression of IL-4, IL-5, and IL-13 and leads to severe pathological changes in mucosal organs.























































 膜杰作
膜杰作 Star Staining
Star Staining















