Redirecting immune signaling with cytokine adaptorsAbhiraman, Householder, Rodriguez
et alNat Commun (2025) 16 (1), 2432
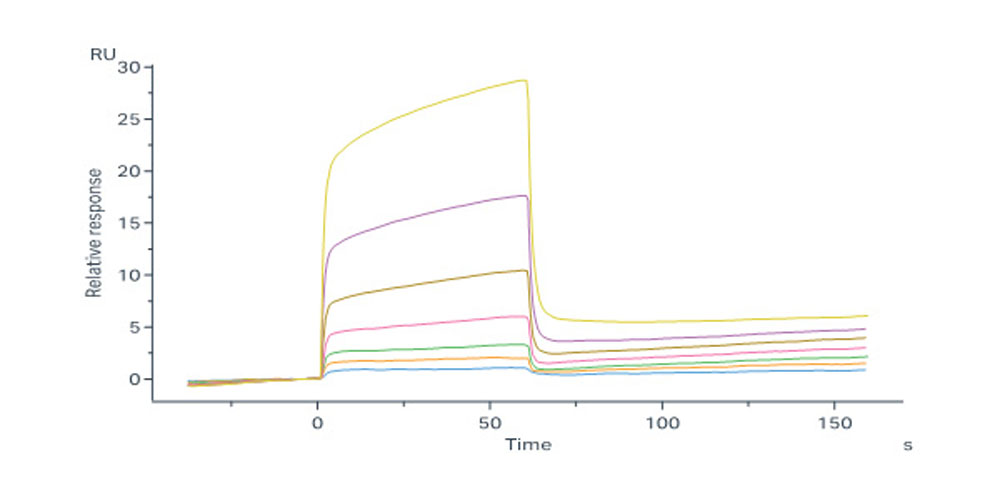
Abstract: Cytokines are signaling molecules that coordinate complex immune processes and are frequently dysregulated in disease. While cytokine blockade has become a common therapeutic modality, cytokine agonism has had limited utility due to the widespread expression of cytokine receptors with pleiotropic effects. To overcome this limitation, we devise an approach to engineer molecular switches, termed cytokine adaptors, that transform one cytokine signal into an alternative signal with a different functional output. Endogenous cytokines act to nucleate the adaptors, converting the cytokine-adaptor complex into a surrogate agonist for a different cytokine pathway. In this way, cytokine adaptors, which have no intrinsic agonist activity, can function as conditional, context-dependent agonists. We develop cytokine adaptors that convert IL-10 or TGF-β into IL-2 receptor agonists to reverse T cell suppression. We also convert the pro-inflammatory cytokines IL-23 or IL-17 into immunosuppressive IL-10 receptor agonists. Thus, we show that cytokine adaptors can convert immunosuppressive cytokines into immunostimulatory cytokines, or vice versa. Unlike other methods of immune conversion that require cell engineering, cytokine adaptors are soluble molecules that leverage endogenous cues from the microenvironment to drive context-specific signaling.© 2025. The Author(s).
G3BP1 Regulates the Cell Cycle by Promoting IFNβ Production to Promote PCV2 Replication and Promotes Nuclear Transfer of Viral Proteins by Direct BindingZhang, Li, Zhou
et alInt J Mol Sci (2025) 26 (3)
Abstract: Porcine circovirus type 2 (PCV2) is a significant pathogen responsible for porcine circovirus-associated diseases (PCVAD), and it is widely prevalent in pig farms, leading to huge economic losses for the pig industry. Currently, the ability of PCV2 to enhance its own replication by using the antiviral inflammatory factors IFNα, IFNβ, and IL-2 and its complex immune escape mechanism remain unclear, which has attracted wide attention. Research has indicated that GTPase-activating protein (SH3 domain)-binding protein 1 (G3BP1) is involved in the innate immune response to a variety of viruses, primarily by regulating and composing stress granules (SGs) to inhibit viral replication. Our initial studies identified elevated G3BP1 expression during PCV2 infection, paradoxically promoting PCV2 replication. In light of this phenomenon, this study aims to elucidate how PCV2 regulates G3BP1 to enhance its replication. Our findings demonstrate that G3BP1 overexpression further activates PCV2-induced expression of RIG-I, MDA5, cGAS and STING, thereby promoting IFNβ production and affecting cell cycle arrest in the S phase, facilitating PCV2 replication. Moreover, interactions were observed between PCV2 Cap protein and G3BP1's RGG domain, and between PCV2 Rep protein and G3BP1's NTF2 and RRM domains, potentially promoting viral protein nuclear transfer. In summary, PCV2 enhances its replication by modulating G3BP1 to induce IFNβ production and directly binds viral proteins to promote viral protein nuclear transfer. This research provides a foundation for further investigation into the immune evasion mechanisms of PCV2.
Elucidating the Causal Dynamics between Inflammatory Proteins and Atrial Fibrillation Risk Through Bidirectional Mendelian RandomizationLv, Huang, Xu
et alCurr Mol Med (2025)
Abstract: Atrial fibrillation (AF), the most common cardiac arrhythmia, is associated with significant morbidity and mortality. Inflammation has been implicated in the pathogenesis of AF, but the causal relationship between specific inflammatory proteins and AF risk is not well established. This study aims to clarify this relationship using a bidirectional two-sample Mendelian Randomization (TSMR) approach.Employing a bidirectional Mendelian Randomization (MR) method, we analyzed genetic variants as instrumental variables (IVs) to investigate the influence of 91 circulating inflammatory proteins on AF risk. This approach allowed us to assess the potential causal effects of inflammatory proteins on AF and vice versa, thus providing a comprehensive understanding of the bidirectional nature of their relationship.Seven inflammatory proteins were significantly associated with AF risk. Three proteins increased the risk: Fibroblast Growth Factor 5 (FGF-5) with an odds ratio (OR) of 1.0743 (95% CI: 1.0466-1.1027, p=7.41E-08), Tumor Necrosis Factor (TNF) with an OR of 1.0832 (95% CI: 1.0261-1.1434, p=0.0038), and Interleukin-2 Receptor Subunit Beta (IL-2RB) with an OR of 1.0814 (95% CI: 1.0151-1.1519, p=0.0153). Four proteins showed a protective effect: CD40 Ligand Receptor (CD40) with an OR of 0.9671 (95% CI: 0.9392-0.9959, p=0.0254), Fms-related Tyrosine Kinase 3 Ligand (FIt3L) with an OR of 0.9553 (95% CI: 0.9173-0.9949, p=0.0274), Leukemia Inhibitory Factor Receptor (LIF-R) with an OR of 0.9254 (95% CI: 0.8678- 0.9868, p=0.0181), and Sulfotransferase 1A1 (ST1A1) with an OR of 0.9461 (95% CI: 0.9097-0.9839, p=0.0056). The reverse MR analysis revealed no significant effects of AF on the levels of these inflammatory proteins, suggesting a unidirectional causality from proteins to AF.This bidirectional MR study provides robust evidence for a causal relationship between specific inflammatory proteins and AF risk. The identified proteins could serve as potential biomarkers for AF risk stratification and targets for therapeutic intervention, offering new insights into the pathophysiology of AF and avenues for future research.Copyright© Bentham Science Publishers; For any queries, please email at epub@benthamscience.net.
Learning from Hindsight: Examining Autonomic, Inflammatory, and Endocrine Stress Biomarkers and Mental Health in Healthy Terrorism Survivors Many Years LaterTucker, Pfefferbaum, North
et alPrehosp Disaster Med (2024) 39 (5), 335-343
Abstract: Terrorism and trauma survivors often experience changes in biomarkers of autonomic, inflammatory and hypothalamic-pituitary-adrenal (HPA) axis assessed at various times. Research suggests interactions of these systems in chronic stress.This unprecedented retrospective study explores long-term stress biomarkers in three systems in terrorism survivors.Sixty healthy, direct terrorism survivors were compared to non-exposed community members for cardiovascular reactivity to a trauma script, morning salivary cortisol, interleukin 1-β (IL-1β), and interleukin 2-R (IL-2R). Survivors' biomarkers were correlated with psychiatric symptoms and diagnoses and reported functioning and well-being seven years after the Oklahoma City (OKC) bombing.Main outcome measures were the Diagnostic Interview Schedule (DIS) Disaster Supplement for Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR) diagnoses, Impact of Events Scale-Revised (IES-R), Beck Depression Inventory-II (BDI-II), Distress and Functioning Scale (DAF), and General Physical Well-Being Scale.Survivors had higher inflammatory IL-1β, lower anti-inflammatory IL-2R, lower cortisol, higher resting diastolic blood pressure (BP), and less cardiovascular reactivity to a trauma script than comparisons. Survivors' mean posttraumatic stress (PTS) symptom levels did not differ from comparisons, but survivors reported worse well-being. None of survivors' biomarkers correlated with PTS or depressive symptoms or diagnoses or reported functioning.Alterations of biological stress measures in cardiovascular, inflammatory, and cortisol systems coexisted as an apparent generalized long-term response to terrorism rather than related to specific gauges of mental health. Potential interactions of biomarkers long after trauma exposure is discussed considering relevant research. Longer-term follow-up could determine whether biomarkers continue to differ or correlate with subjective measures, or if they accompany health problems over time. Given recent international terrorism, understanding long-term sequelae among direct survivors is increasingly relevant.

























































 膜杰作
膜杰作 Star Staining
Star Staining















