Construction and evaluation of recombinant rabies virus encoding three copies codon-optimized G genes as inactivated rabies vaccine in dogs and catsWu, Li, Wang
et alVet Microbiol (2025) 304, 110481
Abstract: Rabies is a fatal zoonotic disease affecting various warm-blooded animals. The number of dogs and cats in China has surpassed 120 million, yet no domestic universal rabies vaccine for them. The glycoprotein (G) of the rabies virus (RABV) serves as the principal antigen for inducing virus-neutralizing antibodies (VNA). In this study, we constructed a recombinant RABV strain (designated SAD-tOG) carrying three copies of the codon-optimized G gene using reverse genetics. The SAD-tOG strain exhibited comparable growth kinetics to the parent strain in cell culture while demonstrating enhanced G protein expression. Mouse challenge experiments revealed significantly reduced pathogenicity and elevated immunogenicity in the recombinant strain. Subsequently, a safe and efficient water-based adjuvant was selected to prepare an inactivated rabies vaccine. In another experiment, the VNA titers of dogs and cats after vaccination were both greater than the WHO-recommended protective antibody titer of 0.5 IU/ml for 360 days. Comparative analysis showed these titers were higher than those induced by commercial vaccines currently used in China. These findings collectively indicate that the SAD-tOG strain represents a safer and more effective vaccine candidate for dog and cat rabies prevention.Copyright © 2025 Elsevier B.V. All rights reserved.
Evaluation of the immune status of dogs vaccinated against rabies by an enzyme-linked immunosorbent assay using crude preparations of insect cells infected with a recombinant baculovirus encoding the rabies virus glycoprotein geneSantosh, Kumar, Kaur
et alPLoS One (2024) 19 (12), e0314516
Abstract: Evaluation of the effectiveness of vaccination of animals against rabies is not routinely implemented. In cases where it is carried out, the rapid fluorescent focus inhibition test (RFFIT) or the fluorescent antibody virus neutralization (FAVN) test are the recommended tests. However, both of these tests require handling of live rabies virus (RABV), and are cumbersome to perform. In view of this, the enzyme-linked immunosorbent assay (ELISA) has been proposed as a surrogate test; however, availability of appropriate antigen is a major impediment for the development of ELISAs to detect anti-rabies antibodies. The most widely used antigen is the RABV glycoprotein (G) purified from cell culture-propagated virus, which requires a biosafety level 3 containment. The alternative is to use recombinantly expressed G, which needs to be to be properly glycosylated and folded to serve as the best antigen. The most suitable system for its production is the baculovirus expression system (BVES). However, purification of RABV G is challenging. We therefore tested partially purified preparations in the form of extracts of insect cells infected with baculovirus expressing RABV G, against sera from vaccinated dogs in an indirect ELISA. The results showed good concordance against RFFIT, with sensitivity and specificity of 90.48% and 80.00%, respectively. The system may be used for quick screening to determine the presence and an approximate level of antibodies, and can be modified to enable monitoring of mass dog vaccination programs, as well as to facilitate certification of dogs intended for international travel and transportation.Copyright: © 2024 Santosh et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Structural insight into rabies virus neutralization revealed by an engineered antibody scaffoldKedari, Iheozor-Ejiofor, Salminen
et alStructure (2024) 32 (12), 2220-2230.e4
Abstract: Host-cell entry of the highly pathogenic rabies virus (RABV) is mediated by glycoprotein (G) spikes, which also comprise the primary target for the humoral immune response. RABV glycoprotein (RABV-G) displays several antigenic sites that are targeted by neutralizing monoclonal antibodies (mAbs). In this study, we determined the epitope of a potently neutralizing human mAb, CR57, which we engineered into a diabody format to facilitate crystallization. We report the crystal structure of the CR57 diabody alone at 2.38 Å resolution, and in complex with RABV-G domain III at 2.70 Å resolution. The CR57-RABV-G structure reveals critical interactions at the antigen interface, which target the conserved "KLCGVL" peptide and residues proximal to it on RABV-G. Structural analysis combined with a cell-cell fusion assay demonstrates that CR57 effectively inhibits RABV-G-mediated fusion by obstructing the fusogenic transitions of the spike protein. Altogether, this investigation provides a structural perspective on RABV inhibition by a potently neutralizing human antibody.Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Analysis of the antigenic and immunogenic properties of the native rabies virus glycoprotein purified by Lens culinaris lectin affinity chromatographyda Silva, Queiroz, Nogi
et alJ Virol Methods (2025) 331, 115044
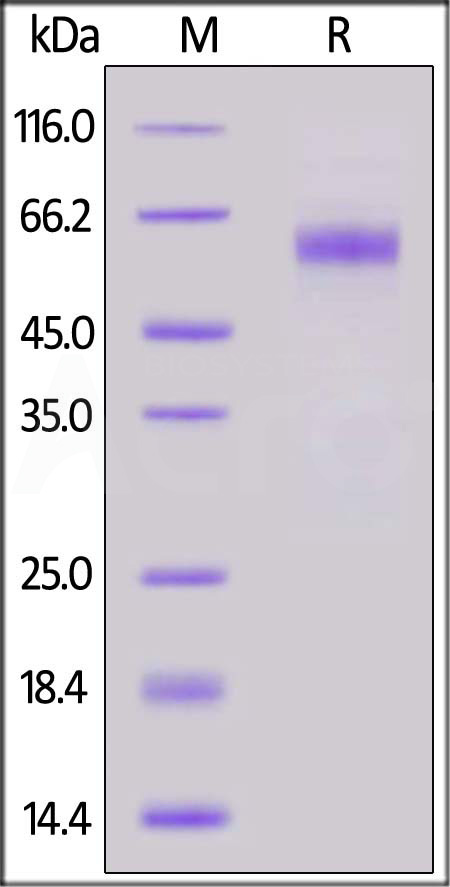
Abstract: Rabies virus glycoprotein (RABV-G) is responsible for the recognition of specific cell surface receptors and induces the production of neutralizing antibodies (VNA). Since RABV-G is a glycoprotein, this work aimed to evaluate Lens culinaris (LCA) chromatography as a simple and effective purification method. The purity and identification of the protein obtained were analyzed by SDS-PAGE, ELISA and lectin-binding assay. The antigenic properties of the purified RABV-G were evaluated by direct ELISA using human serum samples from individuals who had received rabies pre-exposure vaccination. For the immunogenicity study, Swiss Webster mice were immunized with purified RABV-G and the specific antibodies were measured by direct ELISA and RFFIT. As results, it was observed that the purified protein reveled a molecular mass of 55 kDa and the presence of carbohydrate; additionally, it was recognized by anti-rabies virus glycoprotein monoclonal antibody. Purified RABV-G induced high VNA titers (>50.0 IU/ml) in vivo, as detected by RFFIT, as well as RABV-G specific IgG1 (0.8 mean OD±SD) and IgG2a (0.3 mean OD±SD) antibodies, with a predominance of IgG1 (p< 0.001). In addition, it was observed that RABV-G was efficient in selectively detecting anti- RABV-G IgG in the sera of vaccinated individuals compared to the negative control. Therefore, LCA chromatography was efficient in preserving the native properties of RABV-G that are essential in inducing an adequate humoral immune response. In addition, the purified RABV-G presented analytical potential as an ELISA reagent.Copyright © 2024 Elsevier B.V. All rights reserved.
























































 膜杰作
膜杰作 Star Staining
Star Staining















