PK/PD of Positively Charged ADC in MiceChang, Le, Liu
et alPharmaceutics (2025) 17 (3)
Abstract: Background/Objectives: Antibody-drug conjugates (ADCs) show significant promise in oncology but often suffer from a narrow therapeutic window. Introducing a positive charge on the antibody is one proposed strategy to enhance tumor distribution and efficacy of ADC. Accordingly, this study evaluates the pharmacokinetics (PK) and pharmacology of an ADC developed using a positively charged (+5) version of anti-HER2 antibody trastuzumab conjugated with vc-MMAE linker-payload. Methods: A positively charged variant of trastuzumab was generated and conjugated to vc-MMAE. In vitro cytotoxicity assays were performed in cell lines with varying HER2 expression levels: N87 (high), MCF-7 (low), and MDA-MB-468 (non-expressing). In vivo biodistribution of wild-type (WT) and positively charged (+5) ADC was investigated in plasma, tumors, liver, and spleen. A pilot efficacy and toxicity study was also conducted in N87 tumor-bearing mice. Results: The charged ADC showed differential potency and PK behavior compared to the WT ADC. The charged ADC had similar potency in N87 cells but demonstrated ~20-fold and ~60-fold higher potency in MCF-7 and MDA-MB-468 cells. Plasma exposures of all the analytes were found to be reduced following the administration of charged ADC. However, total antibody exposure was found to increase in liver, spleen, and low antigen-expressing MCF-7 tumors. Tumor payload exposures were found to be significantly reduced for the charged ADCs, but liver and spleen displayed higher peak concentrations and increased tissue-to-plasma exposure ratios for the payload, suggesting preferential distribution of ADC with high drug-antibody ratio (DAR) to liver and spleen. Consistent with reduced tumor exposures, charged ADC showed lower efficacy in N87 tumor-bearing mice. No overt toxicity was observed for the charged ADC. Conclusions: Our findings suggest that while positively charged ADCs may be more potent in vitro, their efficacy in vivo may be compromised due to altered PK behavior. Thus, introducing a positive charge into the antibody framework may not be a viable strategy for improving the therapeutic potential of ADCs.
Enfortumab vedotin and pembrolizumab: redefining the standard of care for previously untreated advanced urothelial cancerSternschuss, Rosenberg
Future Oncol (2025)
Abstract: Combination treatment with Enfortumab vedotin (EV), an antibody drug conjugate targeting Nectin-4 with a monomethyl auristatin E (MMAE) payload, and pembrolizumab, a programmed death 1 (PD-1) inhibitor, has become the new standard of care for previously untreated locally advanced or metastatic urothelial carcinoma. In the recently published phase III study, EV-302, EV and pembrolizumab demonstrated improved outcomes compared to platinum-based chemotherapy, including objective response rate, progression free survival, and an unprecedented median overall survival of 33.8 months (versus 15.9 months; hazard ratio for death 0.51; 95% confidence interval 0.43-0.61; p < 0.00001). We reviewed the mechanism of action, clinical efficacy, exploratory biomarkers, and safety profile of EV and pembrolizumab as monotherapies and combination in urothelial cancer.
Use of Spinosum Roentgen Index (S.R.I.) to determine candidacy for middle meningeal artery embolizationSozio, Holliday, Hallman
et alClin Imaging (2025) 121, 110457
Abstract: Middle meningeal artery embolization (MMAe) is a minimally-invasive approach for the treatment of subdural hematomas (SDH). Small vessel caliber and ectopic origin of the middle meningeal artery (MMA) may lead to unsuccessful embolization and/or serious morbidity. Thus, use of an objective scale to pre-procedurally assess candidacy for MMA embolization is an essential component of operative planning.A retrospective analysis of all patients having undergone MMAe across 4 years at a single institution was performed.169 MMAe procedures were performed for SDH, of which 3 were found to have a foramen spinosum diameter, termed the "Spinosum Roentgen Index" (SRI), measuring <2 mm on pre-procedure non-contrast CT Head. A statistically-significant correlation was found between foramen spinosum diameter <2 mm and unsuccessful embolization.Assessment of foramen spinosum diameter (Spinosum Roentgen Index (SRI)) may increase suspicion for aberrant MMA anatomy when measuring <2 mm, which can serve as valuable data in assessing risk versus benefit of MMA embolization, and ultimately minimize unexpected intraprocedural or immediate postprocedural complications.Copyright © 2025. Published by Elsevier Inc.
Prognostic significance of frailty in chronic subdural hematoma: implications for treatment selection in the era of middle meningeal artery embolizationJ Dicpinigaitis, Kocharian, Covell
et alNeuroradiology (2025)
Abstract: Middle meningeal artery embolization (MMAE) as a standalone or adjunctive therapy has emerged as an efficacious and safe treatment for chronic/subacute subdural hematoma (csaSDH). The objective of this study is to compare the prognostic significance of frailty in csaSDH patients treated with MMAE alone or with craniotomy/burr hole (CBH).Hospitalization records were identified in the National Inpatient Sample (2016-2020) and the cohort was stratified by increasing frailty thresholds, quantified by the Risk Analysis Index (RAI). Effect sizes of frailty tiers for poor outcome (defined as non-routine discharge disposition) produced from multivariable logistic regression models and discrimination (c-statistic) were evaluated separately in the MMAE only and CBH sub-cohorts.This analysis identified 13,390 csaSDH hospitalizations, of which 595 (5%) documented treatment with MMAE only. Although all frailty tiers of the categorical RAI were significantly associated with poor outcome in the CBH cohort, lower effect sizes were observed in the MMAE cohort. Discrimination of RAI for poor outcome was significantly greater in the CBH cohort compared to the MMAE only cohort.In comparison to surgical evacuation, frailty demonstrated lower effect sizes and worse discrimination for poor outcomes in patients treated with MMAE, suggesting that frail patients may be more likely to achieve better outcomes following this less invasive therapy. MMAE may be considered as a first-line or standalone treatment in certain patients.© 2025. The Author(s), under exclusive licence to Springer-Verlag GmbH Germany, part of Springer Nature.
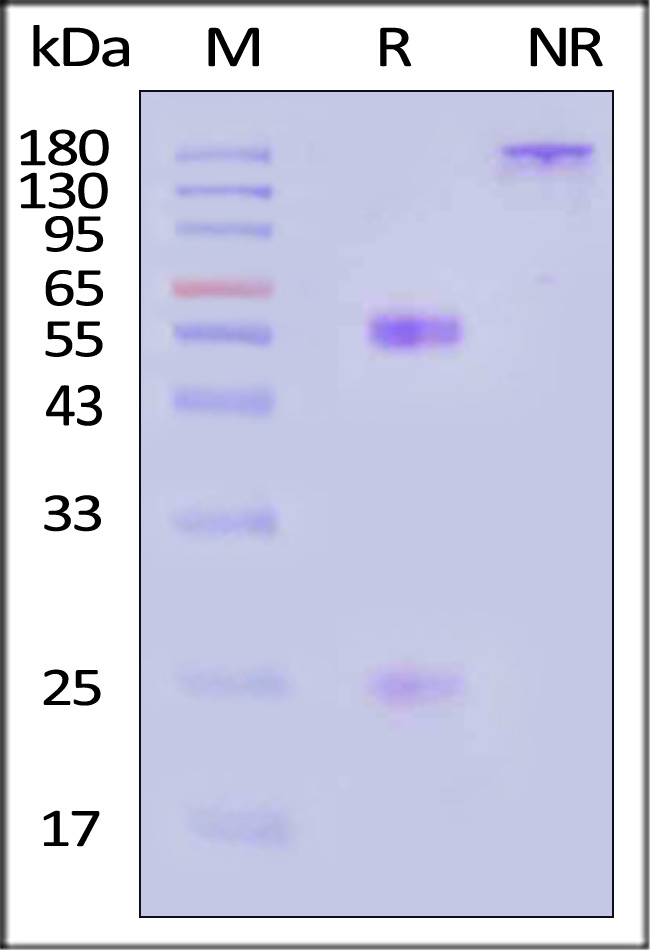
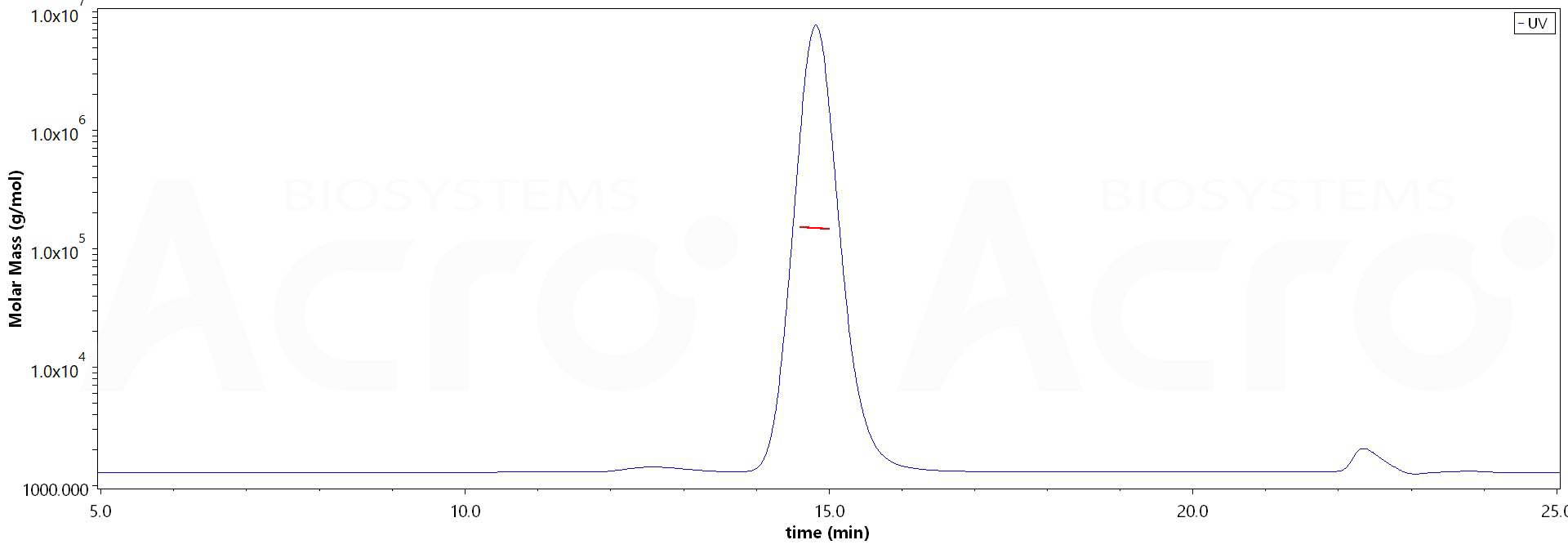
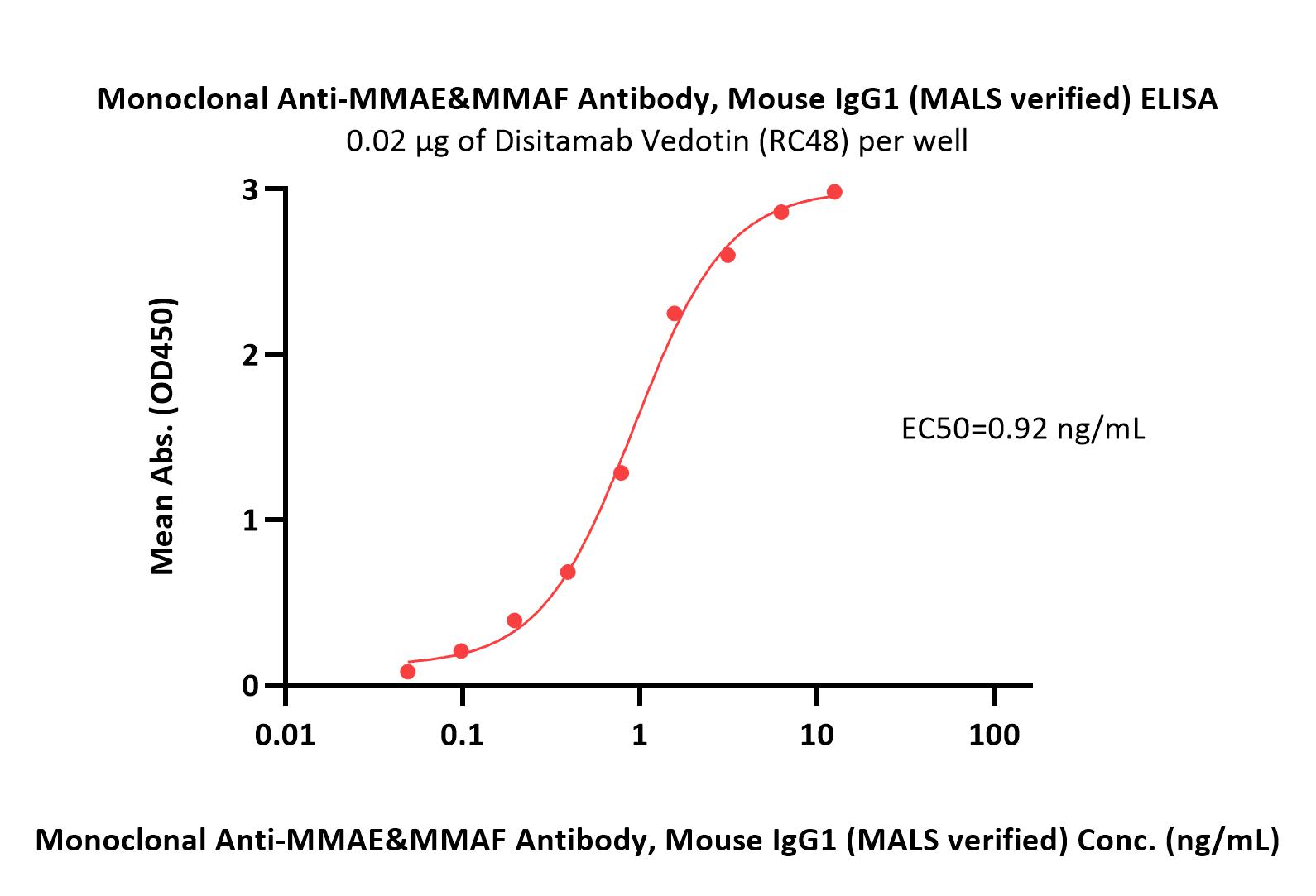
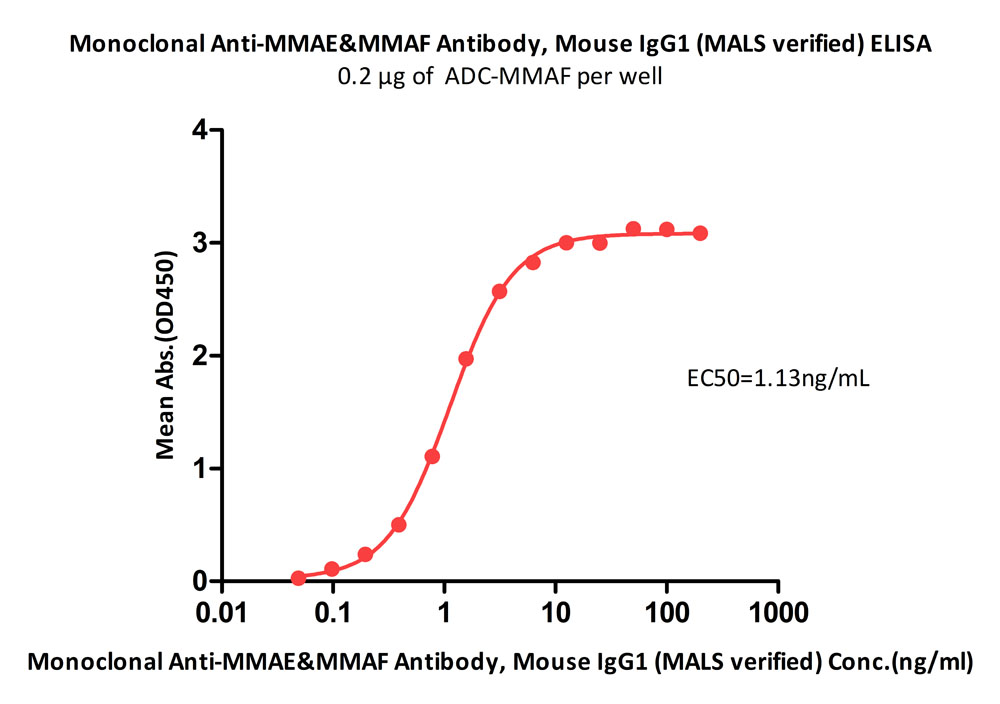
 +添加评论
+添加评论






















































 膜杰作
膜杰作 Star Staining
Star Staining
















