分子别名(Synonym)
ERBB2,CD340,HER-2,neu,HER2,MLN19,NEU,NGL,TKR1
表达区间及表达系统(Source)
FITC-Labeled Human Her2 Protein, Fc Tag (HE2-HF256) is expressed from human 293 cells (HEK293). It contains AA Thr 23 - Thr 652 (Accession # P04626-1).
Predicted N-terminus: Thr 23
Request for sequence
蛋白结构(Molecular Characterization)

This protein carries a human IgG1 Fc tag at the C-terminus
The protein has a calculated MW of 96.0 kDa. The protein migrates as 115-140 kDa under reducing (R) condition (SDS-PAGE) due to glycosylation.
偶联(Conjugate)
FITC
Excitation source: 488 nm spectral line, argon-ion laser
Excitation Wavelength: 488 nm
Emission Wavelength: 535 nm
标记(Labeling)
The primary amines in the side chains of lysine residues and the N-terminus of the protein are conjugated with FITC using standard chemical labeling method. The residual FITC is removed by molecular sieve treatment during purification process.
蛋白标记度(Protein Ratio)
The FITC to protein molar ratio is 1-3.
内毒素(Endotoxin)
Less than 1.0 EU per μg by the LAL method.
纯度(Purity)
>90% as determined by SDS-PAGE.
制剂(Formulation)
Lyophilized from 0.22 μm filtered solution in PBS, pH7.4 with trehalose as protectant.
Contact us for customized product form or formulation.
重构方法(Reconstitution)
Please see Certificate of Analysis for specific instructions.
For best performance, we strongly recommend you to follow the reconstitution protocol provided in the CoA.
存储(Storage)
For long term storage, the product should be stored at lyophilized state at -20°C or lower.
Please protect from light and avoid repeated freeze-thaw cycles.
This product is stable after storage at:
- -20°C to -70°C for 12 months in lyophilized state;
- -70°C for 3 months under sterile conditions after reconstitution.
质量管理控制体系(QMS)
电泳(SDS-PAGE)
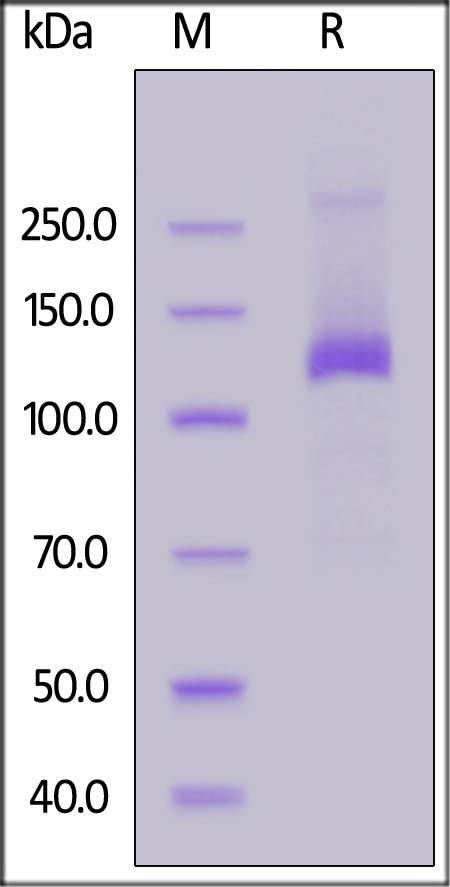
FITC-Labeled Human Her2 Protein, Fc Tag on SDS-PAGE under reducing (R) condition. The gel was stained with Coomassie Blue. The purity of the protein is greater than 90%.
活性(Bioactivity)-ELISA
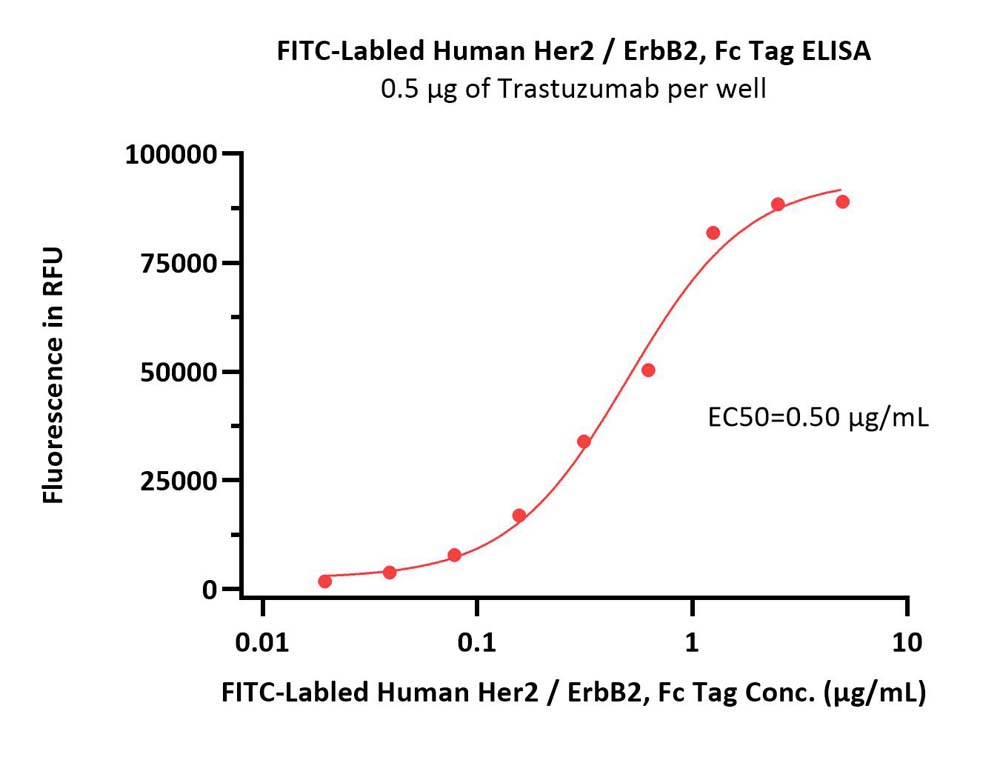
Immobilized Trastuzumab at 5 μg/mL (100 μL/well) can bind FITC-Labeled Human Her2 Protein, Fc Tag (Cat. No. HE2-HF256) with a linear range of 0.02-1.25 μg/mL (QC tested).
Protocol
 +添加评论
+添加评论
- 187XXXXXXX3
- 货期周期短,购买后快速发货,很快就送到,立即用来做PD1的ELISA实验检测的抗原,得到的数据重复性好,结果一致性强,不太容易出现偏差,而且贵公司还有抗原试用装,超级给力,强力推荐该产品
- 2022-10-19
背景(Background)
Human Epidermal growth factor Receptor 2 (HER2) is also called ERBB2, HER-2,HER-2 /neu, NEU, NGL,TKR1 and c-erb B2,and is a protein giving higher aggressiveness in breast cancers. It is a member of the ErbB protein family, more commonly known as the epidermal growth factor receptor family. HER2 is a cell membrane surface-bound receptor tyrosine kinase and is normally involved in the signal transduction pathways leading to cell growth and differentiation. HER2 is thought to be an orphan receptor, with none of the EGF family of ligands able to activate it. Approximately 30% of breast cancers have an amplification of the HER2 gene or overexpression of its protein product. Overexpression of this receptor in breast cancer is associated with increased disease recurrence and worse prognosis. HER2 appears to play roles in development, cancer, communication at the neuromuscular junction and regulation of cell growth and differentiation .























































 膜杰作
膜杰作 Star Staining
Star Staining
















