分子别名(Synonym)
Spike,S protein RBD,Spike glycoprotein Receptor-binding domain,S glycoprotein RBD,Spike protein RBD
表达区间及表达系统(Source)
SARS-CoV-2 Spike RBD, His Tag (B.1.1.529/Omicron) (SPD-C522e) is expressed from human 293 cells (HEK293). It contains AA Arg 319 - Lys 537 (Accession # QHD43416.1 (G339D, S371L, S373P, S375F, K417N, N440K, G446S, S477N, T478K, E484A, Q493R, G496S, Q498R, N501Y, Y505H)). The spike mutations are identified on the SARS-CoV-2 Omicron variant (Pango lineage: B.1.1.529; GISAID clade: GR/484A; Nextstrain clade: 21K).
Predicted N-terminus: Arg 319
Request for sequence
蛋白结构(Molecular Characterization)
This protein carries a polyhistidine tag at the C-terminus.
The protein has a calculated MW of 26.8 kDa. The protein migrates as 33-38 kDa under reducing (R) condition (SDS-PAGE) due to glycosylation.
内毒素(Endotoxin)
Less than 1.0 EU per μg by the LAL method.
纯度(Purity)
>95% as determined by SDS-PAGE.
>90% as determined by SEC-MALS.
制剂(Formulation)
Lyophilized from 0.22 μm filtered solution in PBS, pH7.4 with trehalose as protectant.
Contact us for customized product form or formulation.
重构方法(Reconstitution)
Please see Certificate of Analysis for specific instructions.
For best performance, we strongly recommend you to follow the reconstitution protocol provided in the CoA.
存储(Storage)
For long term storage, the product should be stored at lyophilized state at -20°C or lower.
Please avoid repeated freeze-thaw cycles.
This product is stable after storage at:
- -20°C to -70°C for 12 months in lyophilized state;
- -70°C for 12 months under sterile conditions after reconstitution.
质量管理控制体系(QMS)
电泳(SDS-PAGE)
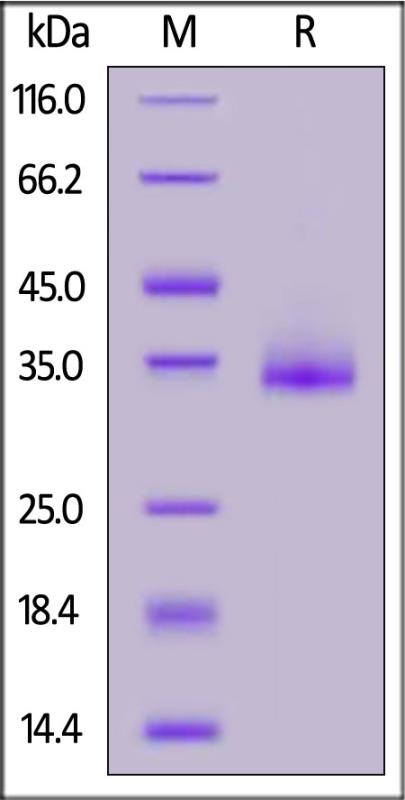
SARS-CoV-2 Spike RBD, His Tag (B.1.1.529/Omicron) on SDS-PAGE under reducing (R) condition. The gel was stained with Coomassie Blue. The purity of the protein is greater than 95%.
SEC-MALS
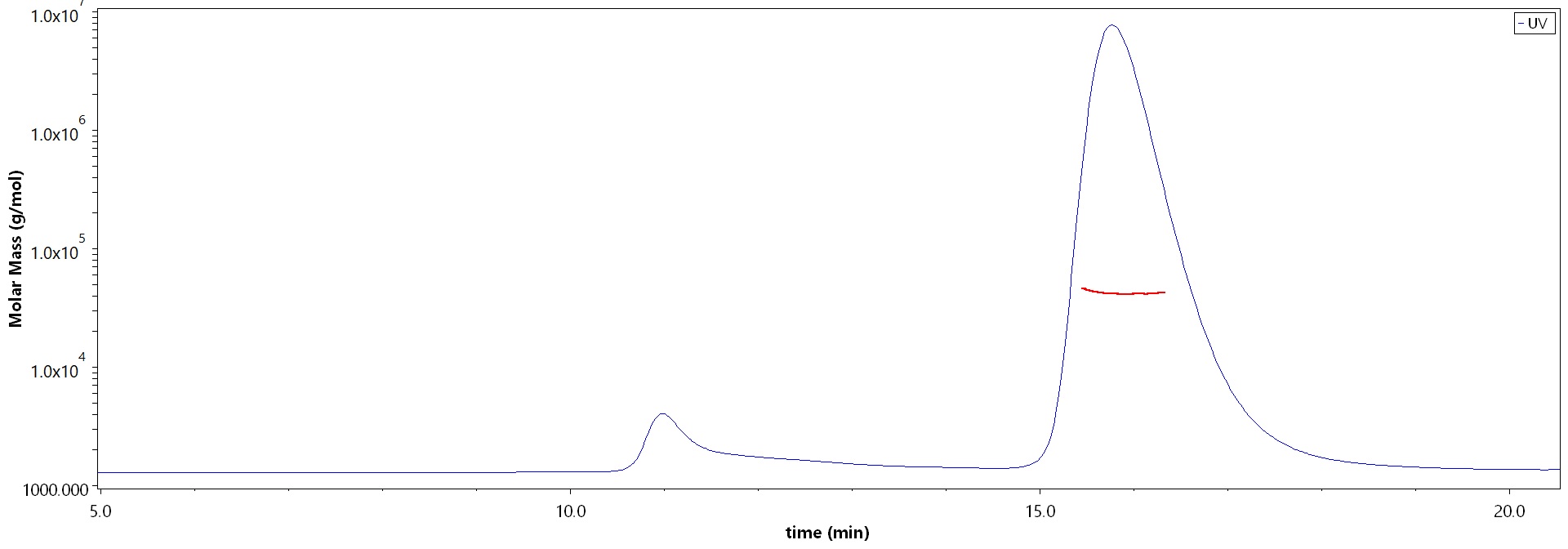
The purity of SARS-CoV-2 Spike RBD, His Tag (B.1.1.529/Omicron) (Cat. No. SPD-C522e) is more than 90% and the molecular weight of this protein is around 33-48 kDa verified by SEC-MALS.
Report
活性(Bioactivity)-ELISA
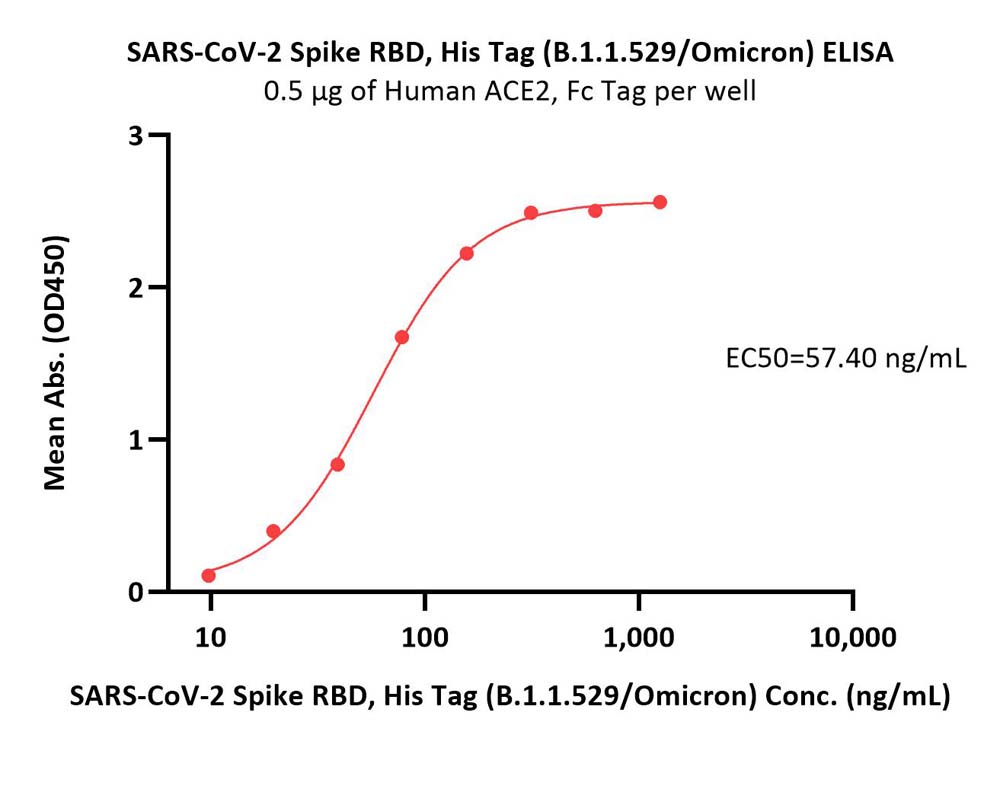
Immobilized Human ACE2, Fc Tag (Cat. No. AC2-H5257) at 5 μg/mL (100 μL/well) can bind SARS-CoV-2 Spike RBD, His Tag (B.1.1.529/Omicron) (Cat. No. SPD-C522e) with a linear range of 10-156 ng/mL (QC tested).
Protocol
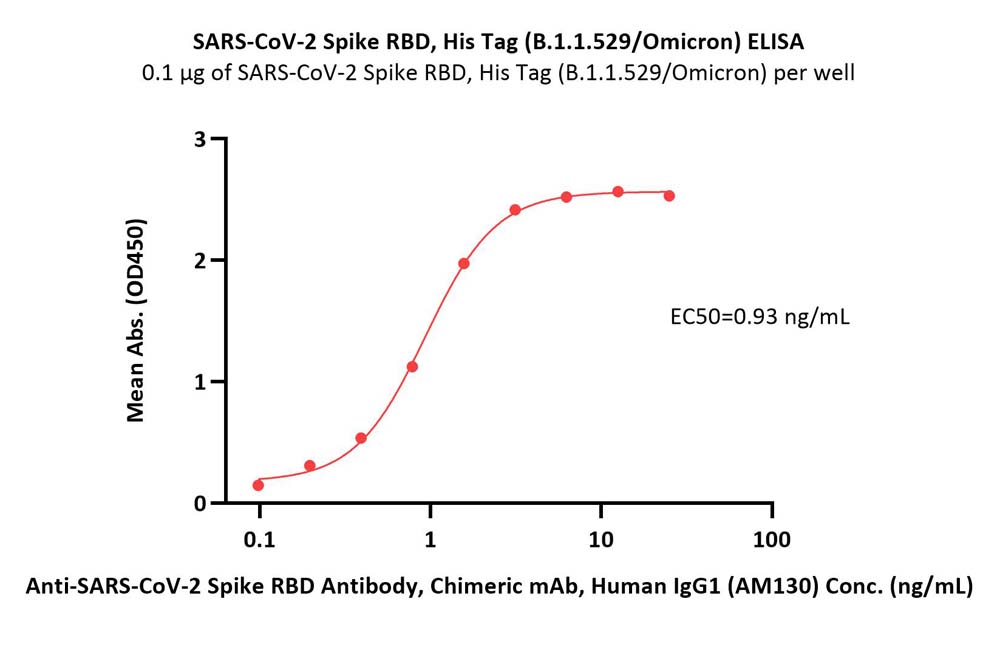
Immobilized SARS-CoV-2 Spike RBD, His Tag (B.1.1.529/Omicron) (Cat. No. SPD-C522e) at 1 μg/mL (100 μL/well) can bind Anti-SARS-CoV-2 Spike RBD Antibody, Chimeric mAb, Human IgG1 (AM130) (Cat. No. S1N-M13A1) with a linear range of 0.1-3 ng/mL (Routinely tested).
Protocol
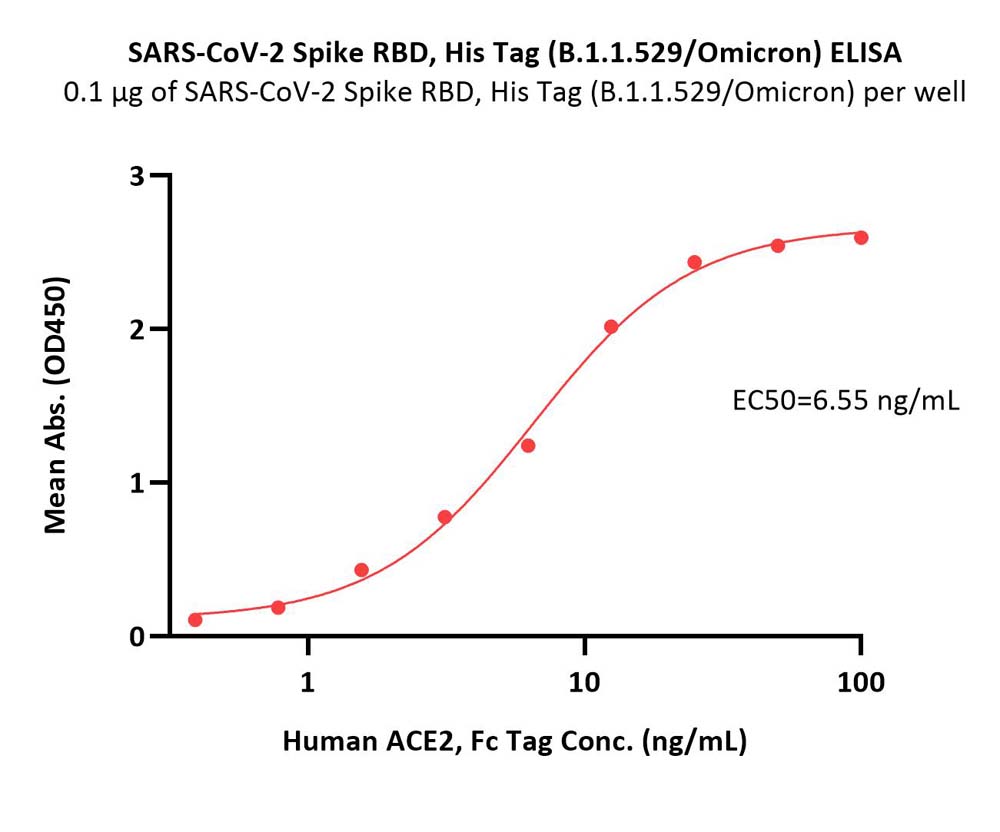
Immobilized SARS-CoV-2 Spike RBD, His Tag (B.1.1.529/Omicron) (Cat. No. SPD-C522e) at 1 μg/mL (100 μL/well) can bind Human ACE2, Fc Tag (Cat. No. AC2-H5257) with a linear range of 0.4-13 ng/mL (Routinely tested).
Protocol
活性(Bioactivity)-SPR
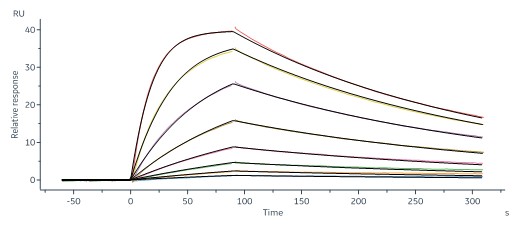
Human ACE2, Fc Tag (Cat. No. AC2-H5257) captured on CM5 chip via human IgG Fc antibody can bind SARS-CoV-2 Spike RBD, His Tag (B.1.1.529/Omicron) (MALS verified) (Cat. No. SPD-C522e) with an affinity constant of 4.56 nM as determined in a SPR assay (Biacore 8K).
Protocol
活性(Bioactivity)-BLI
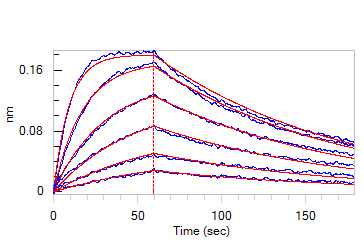
Loaded Human ACE2, Fc Tag (Cat. No. AC2-H5257) on Protein A Biosensor, can bind SARS-CoV-2 Spike RBD, His Tag (B.1.1.529/Omicron) (MALS verified) (Cat. No. SPD-C522e) with an affinity constant of 11.4 nM as determined in BLI assay (ForteBio Octet Red96e).
Protocol
 +添加评论
+添加评论背景(Background)
It's been reported that Coronavirus can infect the human respiratory epithelial cells through interaction with the human ACE2 receptor. The spike protein is a large type I transmembrane protein containing two subunits, S1 and S2. S1 mainly contains a receptor binding domain (RBD), which is responsible for recognizing the cell surface receptor. S2 contains basic elements needed for the membrane fusion.The S protein plays key parts in the induction of neutralizing-antibody and T-cell responses, as well as protective immunity.























































 膜杰作
膜杰作 Star Staining
Star Staining

















