分子别名(Synonym)
Claudin-18.2,CLDN18,Claudin-18
表达区间及表达系统(Source)
Human / Cynomolgus Claudin-18.2, His,Twin-Strep Tag (CL2-H5587) is expressed from Baculovirus-Insect cells. It contains AA Met 1 - Ala 200 (Accession # P56856-2). In the region AA Met 1 - Ala 200, the AA sequence of Human and Cynomolgus Claudin-18.2 are homologus.
Predicted N-terminus: His
Request for sequence
蛋白结构(Molecular Characterization)

This protein carries a polyhistidine tag at the N-terminus and a twin strep tag at the C-terminus.
The protein has a calculated MW of 26.3 kDa.
内毒素(Endotoxin)
Less than 1.0 EU per μg by the LAL method.
纯度(Purity)
>80% as determined by SDS-PAGE.
制剂(Formulation)
This product is not suitable for cell based experiments due to cytotoxicity of DDM.
DDM and CHS are INDISPENSABLE to keep membrane protein soluble and active, under no circumastance should you remove DDM and CHS.
DDM/CHS buffer (DC-11) is sold separately and not included in protein, and please contact us if you need the buffer.
If glycerol is not compatible to your application, remove glycerol just before immediate experiment, and NEVER store glycerol-free protein solution.
Supplied as 0.2 μm filtered solution in 50 mM HEPES, 150 mM NaCl, DDM, CHS, pH7.5 with glycerol as protectant.
Contact us for customized product form or formulation.
运输(Shipping)
This product is supplied and shipped with dry ice, please inquire the shipping cost.
存储(Storage)
Please avoid repeated freeze-thaw cycles.
This product is stable after storage at:
- The product MUST be stored at -70°C or lower upon receipt;
- -70°C for 6 months under sterile conditions.
质量管理控制体系(QMS)
活性(Bioactivity)-ELISA
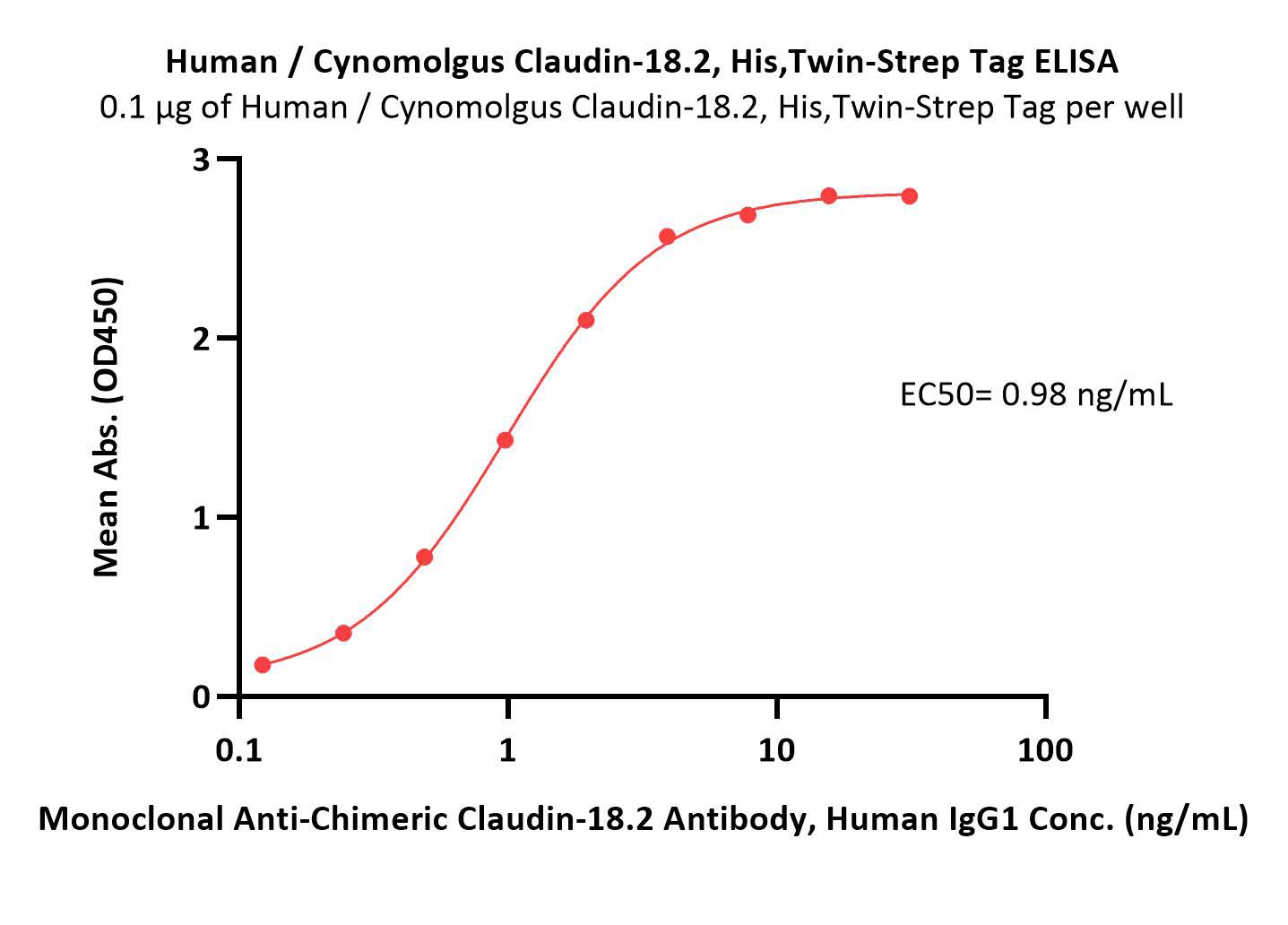
Immobilized Human / Cynomolgus Claudin-18.2, His,Twin-Strep Tag (Cat. No. CL2-H5587) at 1 μg/mL (100 μL/well) can bind Monoclonal Anti-Chimeric Claudin-18.2 Antibody, Human IgG1 (Cat. No. CL2-M34) with a linear range of 0.1-2 ng/mL (QC tested).
Protocol
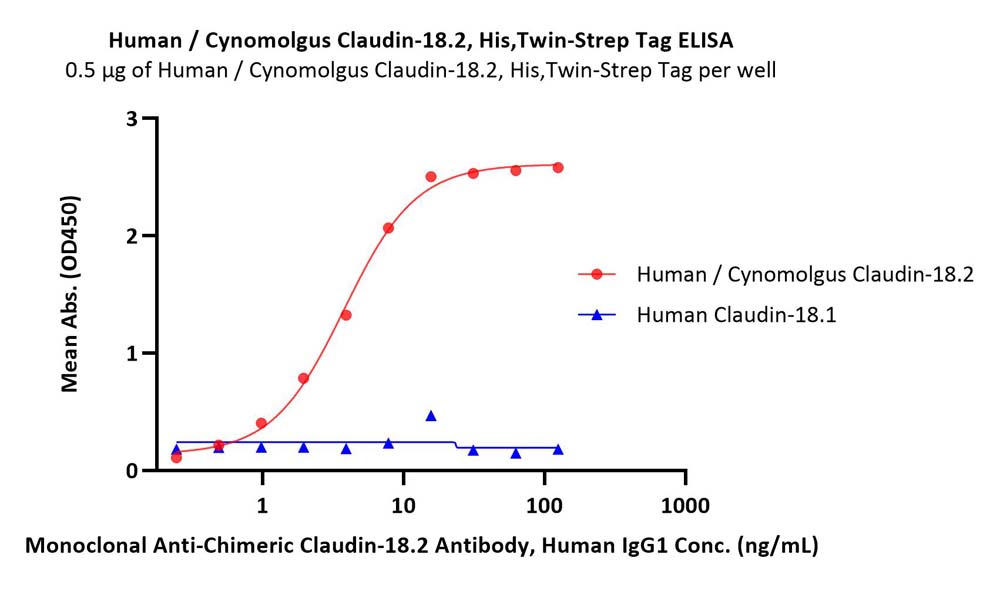
Immobilized Human / Cynomolgus Claudin-18.2, His,Twin-Strep Tag (Cat. No. CL2-H5587) and Human Claudin-18.1, His,Twin-Strep Tag ( Cat. No. CL1-H5588) at 5 μg/mL (100 μL/well) respectively, the Monoclonal Anti-Chimeric Claudin-18.2 Antibody, Human IgG1 can bind the Human / Cynomolgus Claudin-18.2, His,Twin-Strep Tag (Cat. No. CL2-H5587) with a linear range of 0.2-16 ng/mL, but can not bind the Human Claudin-18.1, His,Twin-Strep Tag (Cat. No. CL1-H5588) (Routinely tested).
Protocol
活性(Bioactivity)-SPR
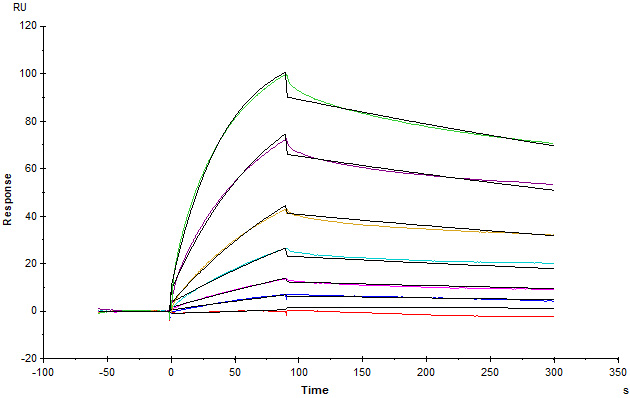
Anti-Claudin-18.2 mAb captured on CM5 chip via anti-Human IgG (Fc) antibody can bind Human / Cynomolgus Claudin-18.2, His,Twin-Strep Tag (Cat. No. CL2-H5587) with an affinity constant of 6.72 nM as determined in a SPR assay (in presence of DDM and CHS) (Biacore T200) (Routinely tested).
Protocol
活性(Bioactivity)-BLI
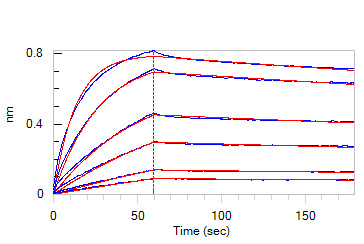
Loaded Monoclonal Anti-Chimeric Claudin-18.2 Antibody, Human IgG1 on AHC Biosensor, can bind Human / Cynomolgus Claudin-18.2, His,Twin-Strep Tag (Cat. No. CL2-H5587) with an affinity constant of 3.03 nM as determined in BLI assay (ForteBio Octet Red96e) (Routinely tested).
Protocol
 +添加评论
+添加评论背景(Background)
Claudins (CLDNs) are a family of proteins that form tight junctions and maintain the polarity of epithelial and endothelial cells. CLDN18 is specifically expressed in the stomach and lung. Of the two CLDN18 isoform transcripts produced by alternative splicing, CLDN18.2 is a highly selective gastric lineage marker that determines the gastric phenotype in a neoplastic condition, whereas CLDN18.1 is lung specific. CLDN18.2 is a highly selective gastric lineage antigen expressed exclusively on short-lived differentiated gastric epithelial cells where it has only limited accessibility to antibody drugs.14,15 CLDN18.2 is maintained during the course of malignant transformation and thus frequently displayed on the surface of human gastric cancer cells.























































 膜杰作
膜杰作 Star Staining
Star Staining














