分子别名(Synonym)
Spike,S protein RBD,Spike glycoprotein Receptor-binding domain,S glycoprotein RBD,Spike protein RBD
表达区间及表达系统(Source)
SARS-CoV-2 Spike RBD, His Tag (BA.2/Omicron) (SPD-C522g) is expressed from human 293 cells (HEK293). It contains AA Arg 319 - Lys 537 (Accession # QHD43416.1 (G339D, S371F, S373P, S375F, T376A, D405N, R408S, K417N, N440K, S477N, T478K, E484A, Q493R, Q498R, N501Y, Y505H). The spike mutations are identified on the SARS-CoV-2 Omicron variant (Pango lineage: BA.2; GISAID clade: GRA; Nextstrain clade: 21L).
Predicted N-terminus: Arg 319
Request for sequence
蛋白结构(Molecular Characterization)
This protein carries a polyhistidine tag at the C-terminus.
The protein has a calculated MW of 26.7 kDa. The protein migrates as 33-40 kDa under reducing (R) condition (SDS-PAGE) due to glycosylation.
内毒素(Endotoxin)
Less than 1.0 EU per μg by the LAL method.
纯度(Purity)
>95% as determined by SDS-PAGE.
>90% as determined by SEC-MALS.
制剂(Formulation)
Lyophilized from 0.22 μm filtered solution in PBS, pH7.4 with trehalose as protectant.
Contact us for customized product form or formulation.
重构方法(Reconstitution)
Please see Certificate of Analysis for specific instructions.
For best performance, we strongly recommend you to follow the reconstitution protocol provided in the CoA.
存储(Storage)
For long term storage, the product should be stored at lyophilized state at -20°C or lower.
Please avoid repeated freeze-thaw cycles.
This product is stable after storage at:
- -20°C to -70°C for 12 months in lyophilized state;
- -70°C for 3 months under sterile conditions after reconstitution.
质量管理控制体系(QMS)
电泳(SDS-PAGE)
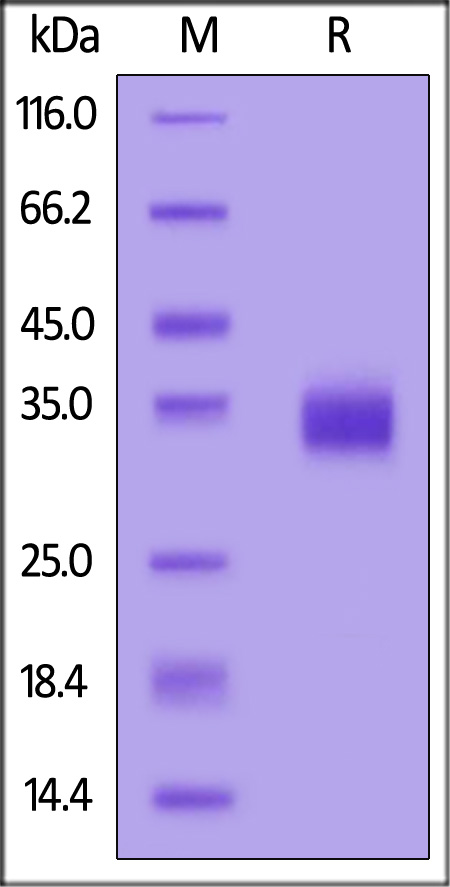
SARS-CoV-2 Spike RBD, His Tag (BA.2/Omicron) on SDS-PAGE under reducing (R) condition. The gel was stained with Coomassie Blue. The purity of the protein is greater than 95%.
SEC-MALS
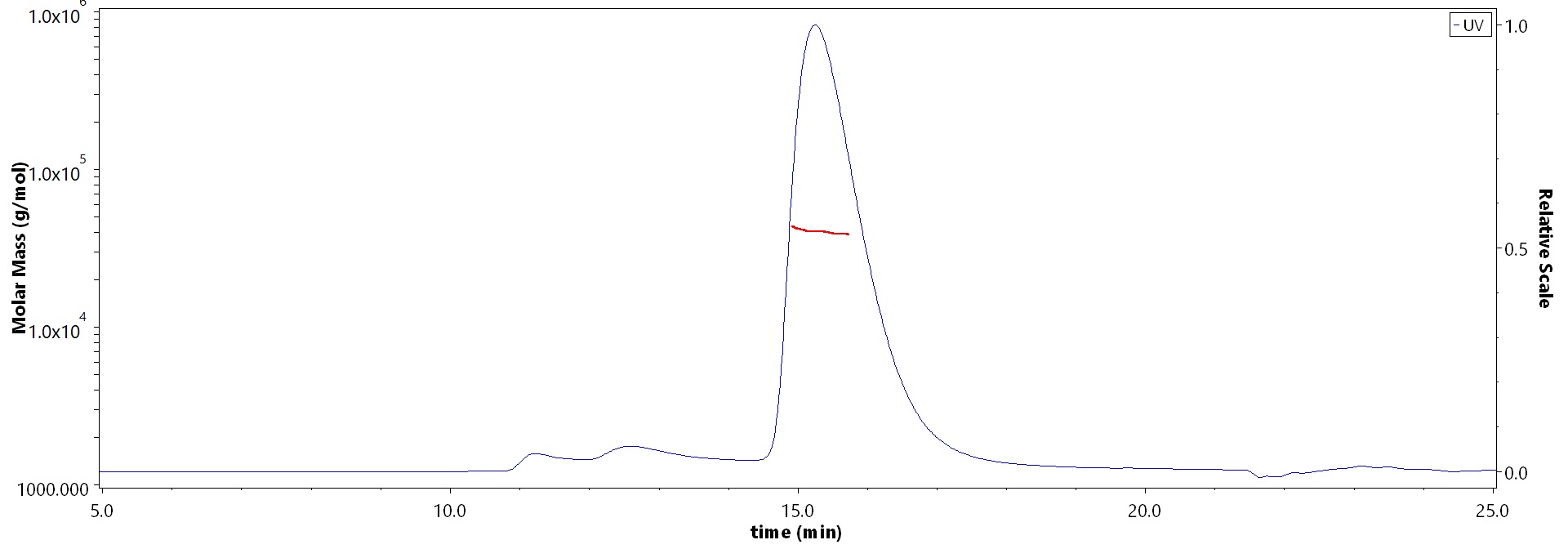
The purity of SARS-CoV-2 Spike RBD, His Tag (BA.2/Omicron) (Cat. No. SPD-C522g) is more than 90% and the molecular weight of this protein is around 32-48 kDa verified by SEC-MALS.
Report
活性(Bioactivity)-ELISA
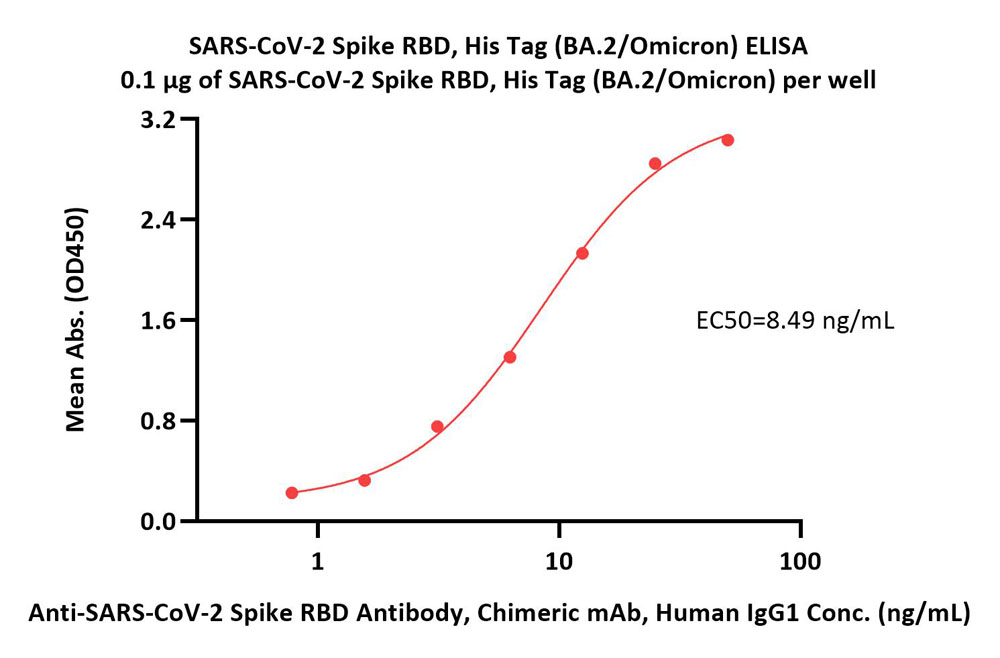
Immobilized SARS-CoV-2 Spike RBD, His Tag (BA.2/Omicron) (Cat. No. SPD-C522g) at 1 μg/mL (100 μL/well) can bind Anti-SARS-CoV-2 Spike RBD Antibody, Chimeric mAb, Human IgG1 (Cat. No. S1N-M122) with a linear range of 0.8-13 ng/mL (QC tested).
Protocol
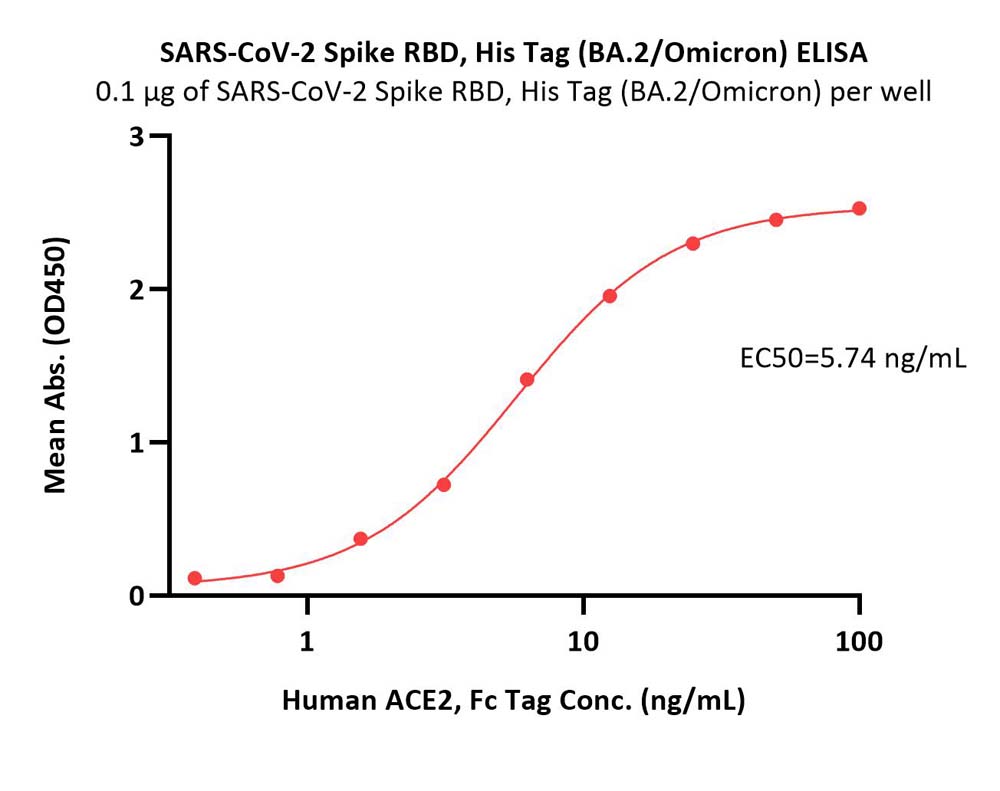
Immobilized SARS-CoV-2 Spike RBD, His Tag (BA.2/Omicron) (Cat. No. SPD-C522g) at 1 μg/mL (100 μL/well) can bind Human ACE2, Fc Tag (Cat. No. AC2-H5257) with a linear range of 0.4-13 ng/mL (Routinely tested).
Protocol
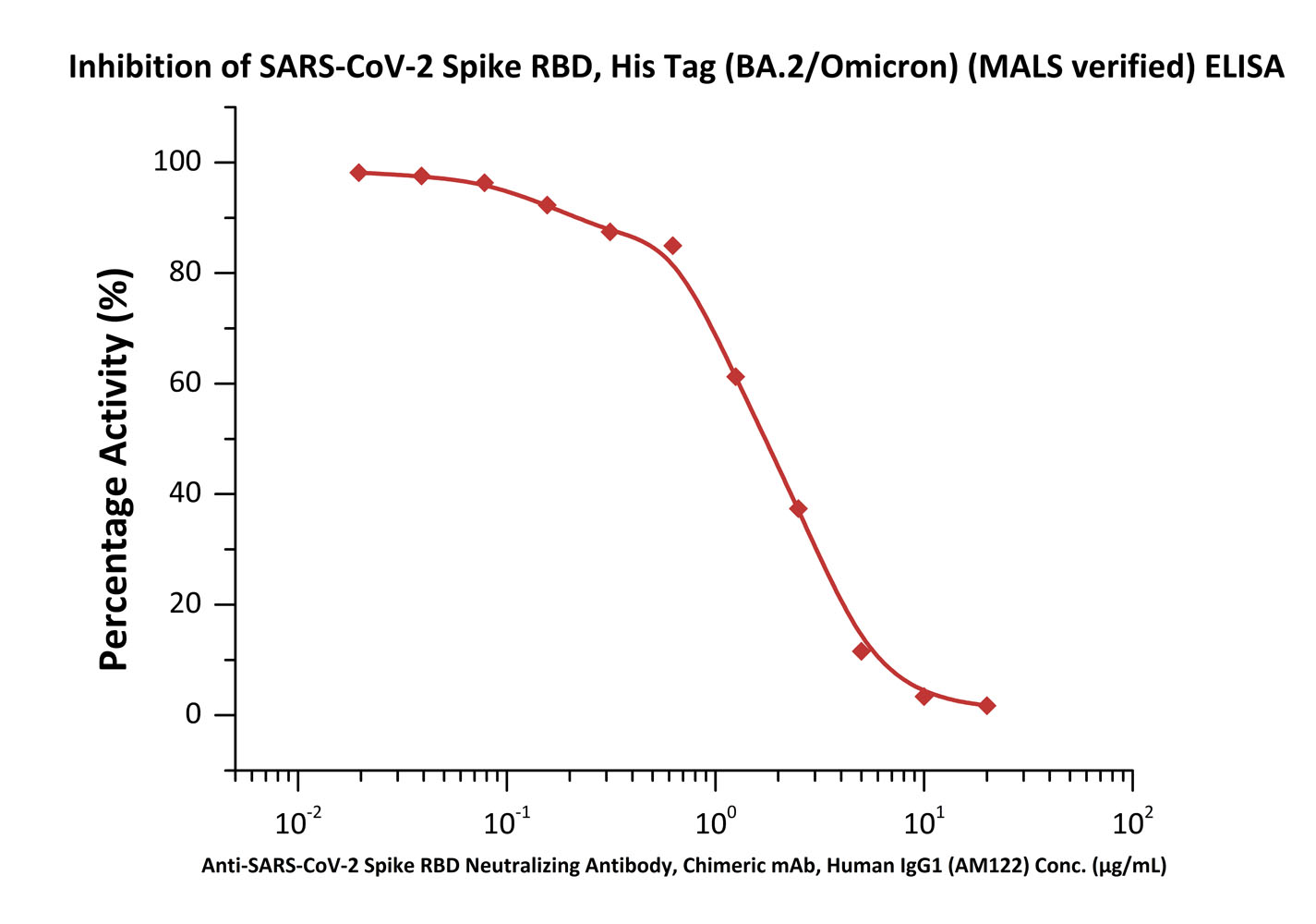
Serial dilutions of Anti-SARS-CoV-2 Spike RBD Neutralizing Antibody, Chimeric mAb, Human IgG1 (AM122) (Trehalose free) (Cat. No. S1N-M12A1) can inhibit SARS-CoV-2 Spike RBD, His Tag (BA.2/Omicron) (MALS verified) (Cat. No. SPD-C522g) from binding Human ACE2. The half maximal inhibitory concentration (IC50) is 1.844 μg/mL (Routinely tested).
Protocol
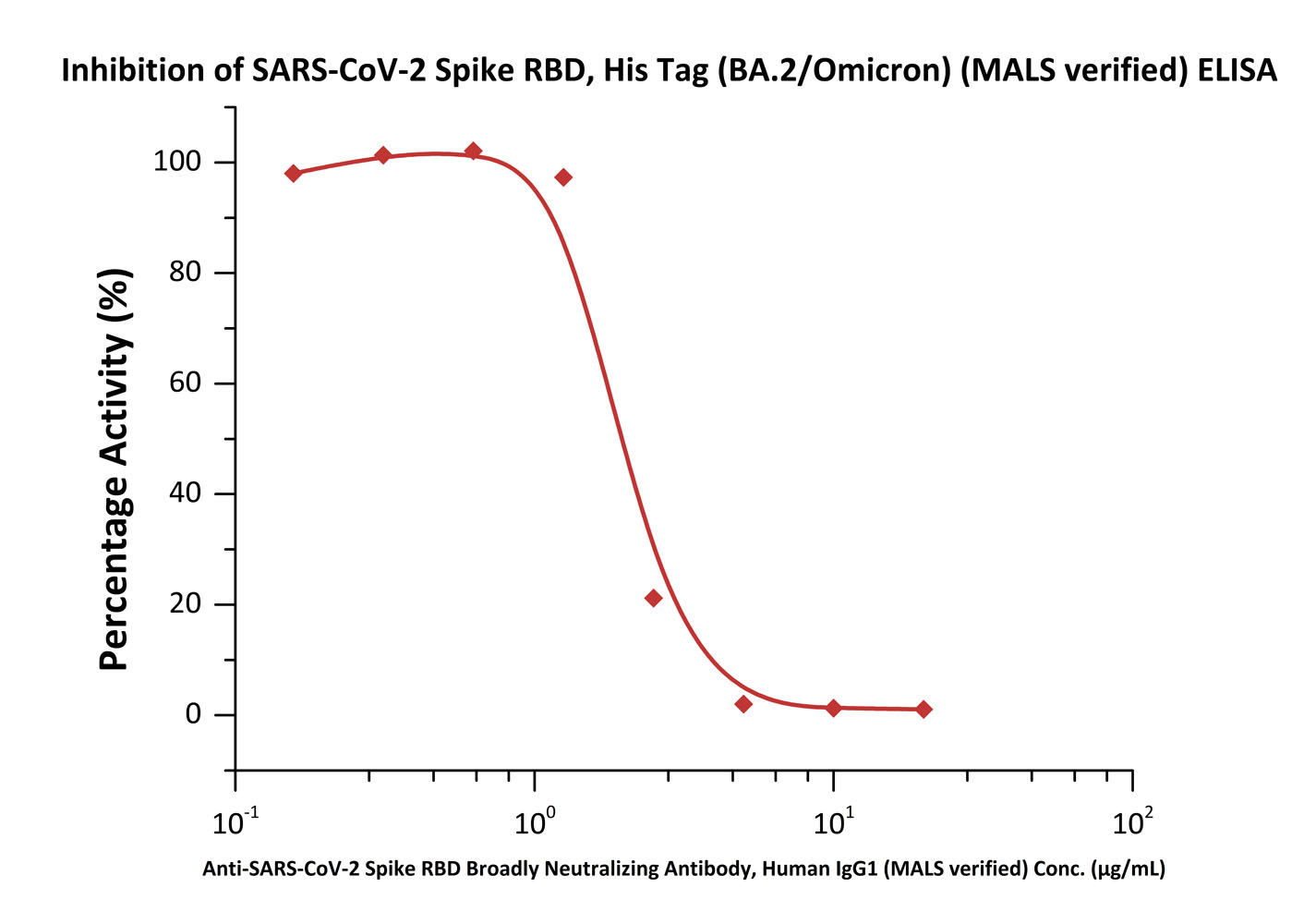
Serial dilutions of Anti-SARS-CoV-2 Spike RBD Broadly Neutralizing Antibody, Human IgG1 (MALS verified) (Cat. No. SPD-M265) can inhibit SARS-CoV-2 Spike RBD, His Tag (BA.2/Omicron) (MALS verified) (Cat. No. SPD-C522g) from binding Human ACE2. The half maximal inhibitory concentration (IC50) is 2.038 μg/mL (Routinely tested).
Protocol
 +添加评论
+添加评论
- 185XXXXXXX6
- 这个蛋白我们是用来包被板子检测抗体含量的,包被板子在4度过夜之后封闭然后加入梯度稀释之后的小鼠血清,最后检测发现抗体滴度都能判断出来,说明蛋白是有效果的,蛋白效价很不错,我们1:3000包被的
 >
>- 2024-4-29

- 188XXXXXXX1
- 计划用来测抗体,该货物发货很快,运送速度很快。卖家服务真周到,非常专业。包装非常仔细、严实,完好无损。实验结果稳定。很满意的一次购物。点个赞!下次再有需要一定再来。
- 2023-6-30
背景(Background)
It's been reported that Coronavirus can infect the human respiratory epithelial cells through interaction with the human ACE2 receptor. The spike protein is a large type I transmembrane protein containing two subunits, S1 and S2. S1 mainly contains a receptor binding domain (RBD), which is responsible for recognizing the cell surface receptor. S2 contains basic elements needed for the membrane fusion.The S protein plays key parts in the induction of neutralizing-antibody and T-cell responses, as well as protective immunity.























































 膜杰作
膜杰作 Star Staining
Star Staining
















