优势特色(Features)
- Designed under ISO 9001:2015 and ISO 13485:2016
- Manufactured and QC tested under a GMP compliance factory
- FDA DMF filed
- Animal-Free materials
- Beta-lactam materials free
- Batch-to-batch consistency
- Stringent quality control tests
表达区间及表达系统(Source)
GMP Human IL-7 Protein (GMP-L07H24) is expressed from human 293 cells (HEK293). It contains AA Asp 26 - His 177 (Accession # P13232-1).
Predicted N-terminus: Asp 26
Request for sequence
蛋白结构(Molecular Characterization)

This protein carries no "tag".
The protein has a calculated MW of 17.4 kDa. The protein migrates as 23 kDa and 28 kDa (±3 kDa) under reducing (R) condition (SDS-PAGE) due to glycosylation.
内毒素(Endotoxin)
Less than 10 EU/mg by the LAL method.
宿主蛋白残留(Host Cell Protein)
<0.5 ng/µg of protein tested by ELISA.
宿主核酸残留(Host Cell DNA)
<0.02 ng/μg of protein tested by DNA Fluorescent Staining method.
无菌(Sterility)
The sterility testing was performed by membrane filtration method described in USP<71> and Ph. Eur. 2.6.1.
支原体(Mycoplasma)
Negative.
外源病毒(In vitro virus assay)
Negative
纯度(Purity)
>95% as determined by SDS-PAGE.
制剂(Formulation)
Lyophilized from 0.22 μm filtered solution in PBS, pH7.4 with protectants.
Contact us for customized product form or formulation.
运输(Shipping)
This product is supplied and shipped with blue ice, please inquire the shipping cost.
存储(Storage)
Upon receipt, store it immediately at -20°C or lower for long term storage.
Please avoid repeated freeze-thaw cycles.
This product is stable after storage at:
- -20°C to -70°C for 5 years in lyophilized state;
- -70°C for 12 months under sterile conditions after reconstitution.
质量管理控制体系(QMS)
电泳(SDS-PAGE)

GMP Human IL-7 Protein on SDS-PAGE under reducing (R) condition. The gel was stained with Coomassie Blue. The purity of the protein is greater than 95%.
活性(Bioactivity)-CELL BASE

GMP Human IL-7 Protein (Cat. No. GMP-L07H24) stimulates proliferation of PHA-P-activated human peripheral blood mononuclear cell (PBMC). The specific activity of GMP Human IL-7 is > 1.00×10^8 IU/mg, which is calibrated against human IL-7 WHO International Standard (NIBSC code: 90/530) (QC tested).
Protocol

The activity of GMP Human IL-7 Protein (Cat. No. GMP-L07H24) was higher than other competing products.
稳定性(Stability)

The Cell based assay shows that GMP Human IL-7 Protein (Cat. No. GMP-L07H24) is stable at 37°C for 48 hours.

The Cell based assay shows that GMP Human IL-7 Protein (Cat. No. GMP-L07H24) is stable at 4℃ for 6 months in 4℃ real time stability experiment.

The Cell based assay shows that GMP Human IL-7 Protein (Cat. No. GMP-L07H24) is stable after freezing and thawing 3 times.
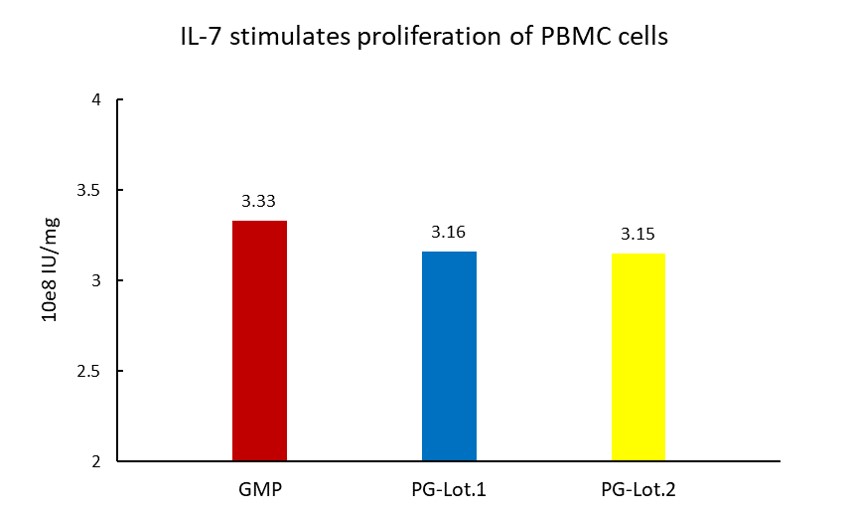
The Cell based assay shows batch-to-batch consistency between Acro's GMP and PG IL-7.
MANUFACTURING SPECIFICATIONS
ACROBiosystems GMP grade products are produced under a quality management system and in compliance with relevant guidelines: Ph. Eur General Chapter 5.2.12 Raw materials of biological origin for the production of cell-based and gene therapy medicinal products; USP <92> Growth Factors and Cytokines Used in Cell Therapy Manufacturing; USP <1043> Ancillary Materials for Cell, Gene, and Tissue-Engineered Products; ISO/TS 20399-1:2018, Biotechnology - Ancillary Materials Present During the Production of Cellular Therapeutic Products.
ACROBiosystems Quality Management System Contents:
- Designed under ISO 9001:2015 and ISO 13485:2016, Manufactured and QC tested under a GMP compliance factory.
- Animal-Free materials
- Materials purchased from the approved suppliers by QA
- ISO 5 clean rooms and automatic filling equipment
- Qualified personnel
- Quality-related documents review and approve by QA
- Fully batch production and control records
- Equipment maintenance and calibration
- Validation of analytical procedures
- Stability studies conducted
- Comprehensive regulatory support files
Request For Regulatory Support Files(RSF) Request For DMF
ACROBiosystems provide rigorous quality control tests (fully validated equipment, processes and test methods) on our GMP grade products to ensure that they meet stringent standards in terms of purity, safety, activity and inter-batch stability, and each bulk QC lot mainly contains the following specific information:
- SDS-PAGE
- Protein content
- Endotoxin level
- Residual Host Cell DNA content
- Residual Host Cell Protein content
- Biological activity analysis
- Microbial testing
- Mycoplasma testing
- In vitro virus assay
- Residual moisture
- Batch-to-batch consistency
DISCLAIMER
ACROBiosystems GMP grade products are designed for research, manufacturing use or ex vivo use. CAUTION: Not intended for direct human use.
TERMS AND CONDITIONS
All products are warranted to meet ACROBiosystems Inc.’s (“ACRO”) published specifications when used under normal laboratory conditions.
ACRO DOES NOT MAKE ANY OTHER WARRANTY OR REPRESENTATION WHATSOEVER, WHETHER EXPRESS OR IMPLIED, WITH RESPECT TO ITS PRODUCTS. IN PARTICULAR, ACRO DOES NOT MAKE ANY WARRANTY OF SUITABILITY, NONINFRINGEMENT, MERCHANTABILITY OR FITNESS FOR ANY PARTICULAR PURPOSE.
NOT WITH STANDING ANY OTHER PROVISIONS OF THESE TERMS AND/OR ANY OTHER AGREEMENT BETWEEN ACRO AND PURCHASER FOR THE PURCHASE OF THE PRODUCTS, ACRO’S TOTAL LIABILITY TO PURCHASER ARISING FROM OR IN RELATION TO THESE TERMS, AN AGREEMENT BETWEEN THE PARTIES OR THE PRODUCTS, WHETHER ARISING IN CONTRACT, TORT OR OTHERWISE SHALL BE LIMITED TO THE TOTAL AMOUNT PAID BY PURCHASER TO ACRO FOR THE RELEVANT PRODUCTS. IN NO EVENT WILL ACRO BE LIABLE FOR THE COST OF PROCUREMENT OF SUBSTITUTE GOODS.
END USER TERMS OF USE OF PRODUCT
The following terms are offered to you upon your acceptance of these End User Terms of Use of Product. By using this product, you indicate your acknowledgment and agreement to these End User Terms of Use of Product. If you do not agree to be bound by and comply with all of the provisions of these End User Terms of Use of Product, you should contact your supplier of the product and make arrangements to return the product.
The End User is aware that ACROBiosystems Inc. and its affiliate (“ACRO”) sell GMP grade products designed for research, manufacturing use or ex vivo use and not intended for human in vivo applications. The End User further agrees, as a condition of the sales of ACRO’s GMP grade products that: a) the End User will not use this GMP grade product in any procedure wherein the product may be directly or indirectly administered to humans, unless the End User has obtained, or prior to their use will have obtained, an Investigational New Drug (IND) exemption from the FDA and will use the product only in accordance with the protocols of such IND and of the Institutional Review Board overseeing the proposed research, or b) the End User will use the products outside of the United States in accordance with the protocols of research approved by the applicable review board or authorized ethics committee and regulatory agencies to which the End User is subject to in their territory.
背景(Background)
Interleukin 7 is also known as IL7, IL-7, and is a hematopoietic growth factor secreted by stromal cells in the red marrow and thymus. It is also produced by keratinocytes, dendritic cells, hepatocytes, neurons, and epithelial cells, but is not produced by lymphocytes. IL-7 stimulates the differentiation of multipotent (pluripotent) hematopoietic stem cells into lymphoid progenitor cells, It also stimulates proliferation of all cells in the lymphoid lineage (B cells, T cells and NK cells). It is important for proliferation during certain stages of B-cell maturation, T and NK cell survival, development and homeostasis. IL-7 is a cytokine important for B and T cell development. This cytokine and the hepatocyte growth factor (HGF) form a heterodimer that functions as a pre-pro-B cell growth-stimulating factor. IL-7 binds to the IL-7 receptor, a heterodimer consisting of Interleukin-7 receptor alpha and common gamma chain receptor. Il-7 promotes hematological malignacies (acute lymphoblastic leukemia, T cell lymphoma). Elevated levels of IL-7 have also been detected in the plasma of HIV-infected patients. IL-7 as an immunotherapy agent has been examined in many pre-clinical animal studies and more recently in human clinical trials for various malignancies and during HIV infection. IL-7 could also be beneficial in improving immune recovery after allogenic stem cell transplant.























































 膜杰作
膜杰作 Star Staining
Star Staining
















