分子别名(Synonym)
IL-23 alpha & IL-12 beta
表达区间及表达系统(Source)
Human IL-23A&IL-12B Heterodimer Protein, premium grade (ILB-H5219) is expressed from human 293 cells (HEK293). It contains AA Ile 23 - Ser 328 (IL-12B) & Arg 20 - Pro 189 (IL-23A) (Accession # P29460-1 (IL-12B) & Q9NPF7-1 (IL-23A)).
Predicted N-terminus: Ile 23
It is produced under our rigorous quality control system that incorporates a comprehensive set of tests including sterility and endotoxin tests. Product performance is carefully validated and tested for compatibility for cell culture use or any other applications in the early preclinical stage. When ready to transition into later clinical phases, we also offer a custom GMP protein service that tailors to your needs. We will work with you to customize and develop a GMP-grade product in accordance with your requests that also meets the requirements for raw and ancillary materials use in cell manufacturing of cell-based therapies.
Request for sequence
蛋白结构(Molecular Characterization)
This protein carries no "tag".
The protein has a calculated MW of 55.0 kDa. The protein migrates as 58 kDa±3 kDa under reducing (R) condition, and 58 kDa when calibrated against Star Ribbon Pre-stained Protein Marker under non-reducing (NR) condition (SDS-PAGE) due to glycosylation.
内毒素(Endotoxin)
Less than 0.1 EU per μg by the LAL method.
无菌(Sterility)
Negative
支原体(Mycoplasma)
Negative.
纯度(Purity)
>95% as determined by SDS-PAGE.
制剂(Formulation)
Lyophilized from 0.22 μm filtered solution in PBS, pH7.4 with trehalose as protectant.
Contact us for customized product form or formulation.
重构方法(Reconstitution)
Please see Certificate of Analysis for specific instructions.
For best performance, we strongly recommend you to follow the reconstitution protocol provided in the CoA.
存储(Storage)
For long term storage, the product should be stored at lyophilized state at -20°C or lower.
Please avoid repeated freeze-thaw cycles.
This product is stable after storage at:
- -20°C to -70°C for 24 months in lyophilized state;
- -70°C for 3 months under sterile conditions after reconstitution.
质量管理控制体系(QMS)
电泳(SDS-PAGE)

Human IL-23A&IL-12B Heterodimer Protein, premium grade on SDS-PAGE under reducing (R) and non-reducing (NR) conditions. The gel was stained with Coomassie Blue. The purity of the protein is greater than 95% (With Star Ribbon Pre-stained Protein Marker).
SEC-MALS

The purity of Human IL-23A&IL-12B Heterodimer Protein, premium grade (Cat. No. ILB-H5219) is more than 85% and the molecular weight of this protein is around 50-70 kDa verified by SEC-MALS.
Report
活性(Bioactivity)-CELL BASE
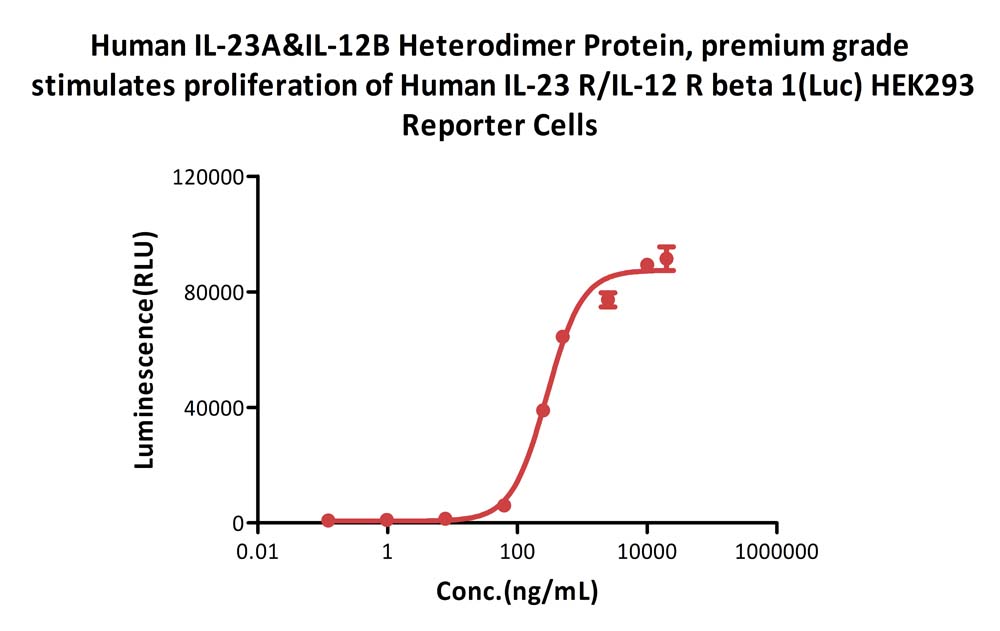
Human IL-23A&IL-12B Heterodimer Protein, premium grade (Cat. No. ILB-H5219) stimulates proliferation of Human IL-23 R/IL-12 R beta 1(Luc) HEK293 Reporter Cell. The specific activity of Human IL-23A&IL-12B Heterodimer Protein, premium grade is > 1.80 X 10^3 U/mg (QC tested).
Protocol
活性(Bioactivity)-ELISA

Immobilized Human IL-23A&IL-12B Heterodimer Protein, premium grade (Cat. No. ILB-H5219) at 1 μg/mL (100 μL/well) can bind Monoclonal Anti-Human IL23A&IL12B P40 domain Antibody, Human IgG1 with a linear range of 0.02-1 ng/mL (QC tested).
Protocol

Immobilized Human IL-23A&IL-12B Heterodimer Protein, premium grade (Cat. No. ILB-H5219) at 5 μg/mL (100 μL/well) can bind Human IL-12 R beta 1, Fc Tag (Cat. No. ILB-H5255) with a linear range of 0.2-3 ng/mL (Routinely tested).
Protocol
 +添加评论
+添加评论背景(Background)
Interleukin-23 subunit alpha (IL-23 alpha) can associates with IL12B to form the IL-23 interleukin, a heterodimeric cytokine which functions in innate and adaptive immunity. IL-23 may constitute with IL-17 an acute response to infection in peripheral tissues. IL-23 binds to a heterodimeric receptor complex composed of IL12RB1 and IL23R, activates the Jak-Stat signaling cascade, stimulates memory rather than naive T-cells and promotes production of proinflammatory cytokines. IL-23 induces autoimmune inflammation and thus may be responsible for autoimmune inflammatory diseases and may be important for tumorigenesis.























































 膜杰作
膜杰作 Star Staining
Star Staining















