A highly stable lyophilized mRNA vaccine for Herpes Zoster provides potent cellular and humoral responsesMunoz-Moreno, Allaj, Gadee
et alNPJ Vaccines (2025) 10 (1), 49
Abstract: Herpes zoster (HZ) is a painful vesicular rash that occurs upon varicella-zoster virus (VZV) reactivation in older adults and immunocompromised individuals. Although there is currently an approved vaccine for the prevention of shingles, its administration is commonly associated with high reactogenicity. This highlights the need to develop new vaccine alternatives with long lasting immunity and improved tolerability upon administration. In the present study, 10 different vaccine candidate designs using two different codon optimizations targeting the VZV glycoprotein E (gE) were generated. A subset of mRNA constructs were formulated into lipid nanoparticles and assessed for their ability to induce specific cellular and humoral immune responses following vaccination in mice. Notably, the selected mRNA vaccine candidates induced high levels of antibodies and robust CD4+ but also CD8+ immune responses. Moreover, we showed that our alternate lyophilized vaccine provides comparable immunogenicity to current liquid frozen formulations and is stable under long-term storage conditions.© 2025. The Author(s).
Immune response and safety of the adjuvanted recombinant zoster vaccine in adults 50 years of age and older in India: A randomized phase 3 trialNaficy, Chugh, Tariq
et alVaccine (2025) 50, 126819
Abstract: Reactivation of latent varicella-zoster virus can cause herpes zoster (shingles) and associated complications, such as post-herpetic neuralgia. The adjuvanted recombinant zoster vaccine (RZV) was shown to be efficacious in preventing herpes zoster and have an acceptable safety profile in adults ≥50 years of age. However, no clinical data on RZV were available in an Indian population. The aim of the current study was to assess the immunogenicity and safety of RZV in adults ≥50 years of age in India.In this randomized, placebo-controlled, observer-blind, multi-center trial, conducted between February 2022 and March 2023, participants ≥50 years of age received two doses (with a two-month interval) of RZV (N = 143) or placebo (N = 145). Blood samples were collected pre-dose 1 and one month post-dose 2 to quantify anti-glycoprotein E (gE) antibody concentrations. Solicited adverse events (AEs) with onset within seven days and unsolicited AEs with onset within 30 days following any RZV or placebo dose were recorded. Serious AEs (SAEs) and potential immune-mediated diseases (pIMDs) were recorded until trial end (six months post-dose 2).At one month post-dose 2, vaccine response rate in the RZV group was 85.7 % (95 % confidence interval [CI]: 78.4 %-91.3 %), meeting the primary objective's success criterion (lower limit of 95 % CI ≥60 %). The adjusted geometric mean anti-gE antibody concentration ratio between the RZV and placebo groups was 19.8 (95 % CI, 14.1-27.8), meeting the secondary objective's success criterion (lower limit of 95 % CI ≥3). Solicited AEs were reported by 103 (72.0 %) RZV and 86 (59.3 %) placebo recipients; most had mild-to-moderate severity. No intervention-related unsolicited AE or SAE and no pIMD or death were reported.Two doses of RZV induced a robust antibody response, comparable to that reported in other populations, and had a safety profile similar to the known RZV safety profile.gov: NCT05219253.Copyright © 2025 GSK. Published by Elsevier Ltd.. All rights reserved.
Immune Response to an Adjuvanted Recombinant Zoster Vaccine in Japanese Patients with Rheumatoid Arthritis Receiving Upadacitinib (End Zoster-J Study): Study Protocol for an Exploratory Parallel Triple-Arm Prospective TrialWatanabe, Fujii, Imai
et alJ Clin Med (2024) 13 (23)
Abstract: Background: Janus kinase (JAK) inhibitors have emerged as a new class of disease-modifying anti-rheumatic drugs in the treatment of rheumatoid arthritis (RA). However, herpes zoster is one of the common adverse events of JAK inhibitors, including upadacitinib, which is especially high in Japanese patients with RA compared to those from Western countries. Recombinant zoster vaccine (Shingrix®) is an adjuvanted subunit vaccine containing varicella-zoster virus (VZV) glycoprotein E (gE) that is effective in adults over 50 years of age. Despite this, no studies have examined its immunogenicity in Japanese patients receiving upadacitinib. Therefore, this study aims to examine the effectiveness of the recombinant zoster vaccine in Japanese patients with RA receiving upadacitinib. Methods: This is a single-center, exploratory, interventional, open-label, parallel triple-arm, prospective study. A total of 69 patients (23 in each group) aged 50 years or over and treated with a stable dose of methotrexate (MTX) monotherapy (6-12 mg/week), upadacitinib monotherapy (15 mg/day), or MTX (6-12 mg/week) + upadacitinib 15 mg/day (combination) for at least 1 month prior to study entry will be included. Moreover, all three groups will receive two intramuscular injections of the recombinant zoster vaccine at 8-week intervals. The primary endpoint is the proportion of positive anti-gE antibodies 4 weeks after the second injection. Secondary endpoints include RA disease activity, positive gE-specific CD4+ T-cells, and VZV-specific antibodies at indicated time points. Data on outcome measures will be collected at baseline and at 4, 8, 12, and 20 weeks. Endpoints will be summarized using descriptive statistics from baseline therapy, and results will be compared in an exploratory manner. Discussion: Despite the limited generalizability due to its design as a single-center, single-ethnic study, small sample size, and short observation period, this study provides evidence on the effectiveness and tolerability of recombinant zoster vaccine in Japanese patients with RA receiving upadacitinib.
Structures of the Varicella Zoster Virus Glycoprotein E and Epitope Mapping of Vaccine-Elicited AntibodiesHarshbarger, Holzapfel, Seraj
et alVaccines (Basel) (2024) 12 (10)
Abstract: Background: Varicella zoster virus (VZV) is the causative agent for chickenpox and herpes zoster (HZ, shingles). HZ is a debilitating disease affecting elderly and immunocompromised populations. Glycoprotein E (gE) is indispensable for viral replication and cell-to-cell spread and is the primary target for anti-VZV antibodies. Importantly, gE is the sole antigen in Shingrix, a highly efficacious, AS01B-adjuvanted vaccine approved in multiple countries for the prevention of HZ, yet the three-dimensional (3D) structure of gE remains elusive. Objectives: We sought to determine the structure of VZV gE and to understand in detail its interactions with neutralizing antibodies. Methods: We used X-ray crystallography and cryo-electron microscopy to elucidate structures of gE bound by recombinant Fabs of antibodies previously elicited through vaccination with Zostavax, a live, attenuated vaccine. Results: The 3D structures resolve distinct central and C-terminal antigenic domains, presenting an array of diverse conformational epitopes. The central domain has two beta-sheets and two alpha helices, including an IgG-like fold. The C-terminal domain exhibits 3 beta-sheets and an Ig-like fold and high structural similarity to HSV1 gE. Conclusions: gE from VZV-infected cells elicits a human antibody response with a preference for the gI binding domain of gE. These results yield insights to VZV gE structure and immunogenicity, provide a framework for future studies, and may guide the design of additional herpesvirus vaccine antigens. Teaser: Structures of varicella zoster virus glycoprotein E reveal distinct antigenic domains and define epitopes for vaccine-elicited human antibodies.
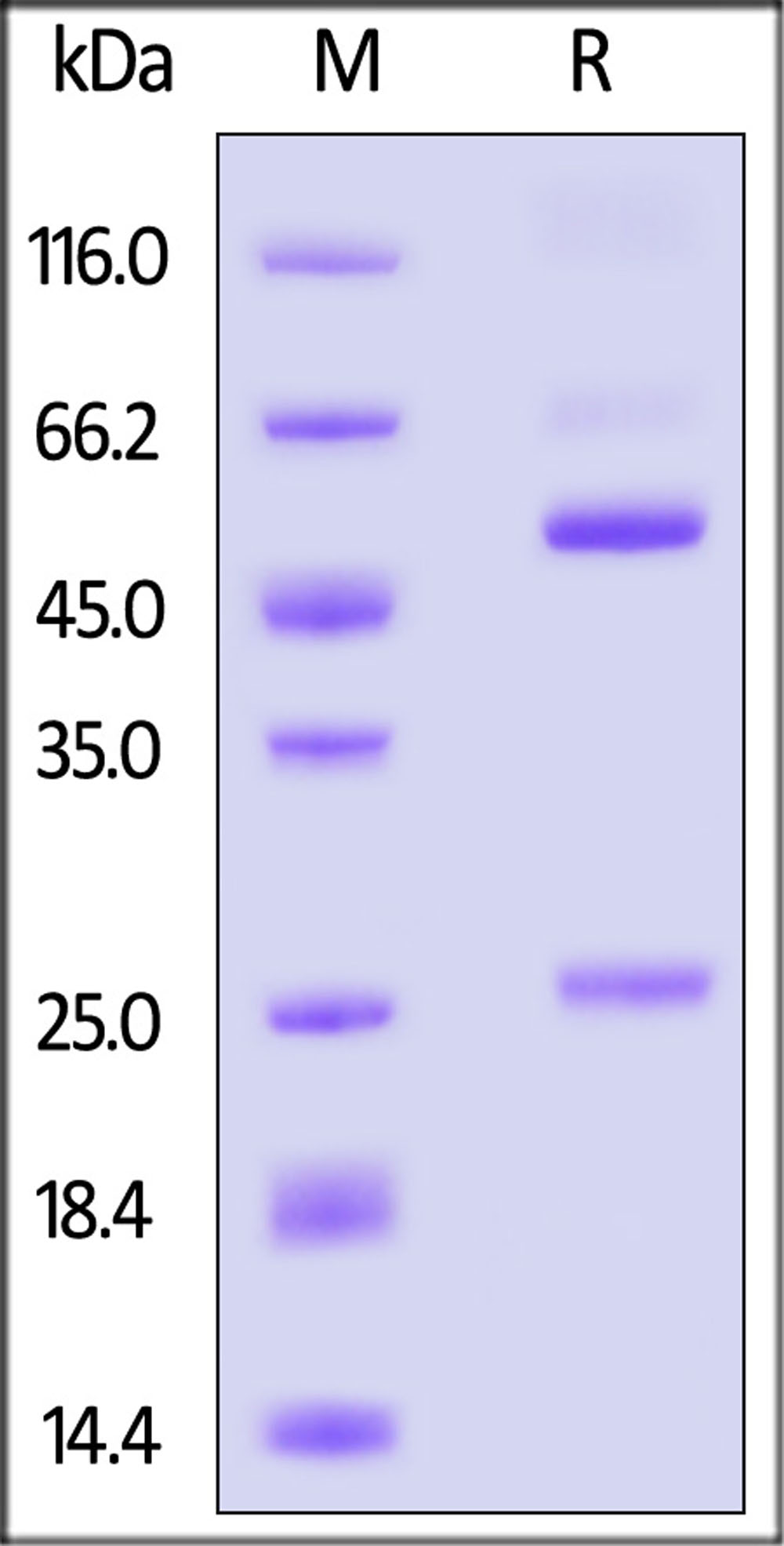



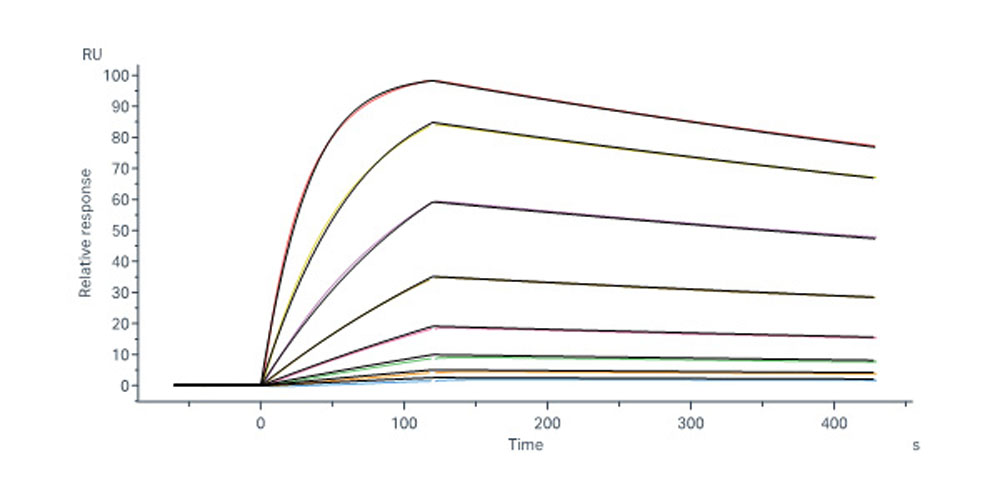
 +添加评论
+添加评论






















































 膜杰作
膜杰作 Star Staining
Star Staining


















