[Intestinal fibrosis in Crohn's disease : towards new therapeutic options?]Vieujean, Bequet, Stepniak
et alRev Med Liege (2025) 80 (3), 154-161
Abstract: Crohn's disease is a chronic inflammatory bowel disease that can lead to fibrostenotic complications. These strictures result from an imbalance between inflammation and excessive healing, leading to an abnormal accumulation of extracellular matrix and a progressive thickening of the intestinal wall. To date, no specific treatment is available to prevent or reverse intestinal fibrosis. The management of strictures primarily focuses on controlling inflammation and addressing obstructive complications through endoscopic balloon dilation, stricturoplasty, or intestinal resection. Current medical therapies, such as anti-TNF agents, can help reduce inflammation but have no direct impact on fibrosis. New therapeutic strategies are emerging, including TL1A inhibitors (duvakitug, tulisokibart), an ALK5 inhibitor (AGMB-129), and targeted AGR2 therapies (TH-009). Additionally, mesenchymal stem cells are being investigated in our hospital center in combination with endoscopic balloon dilation, aiming to assess their therapeutic potential. The development of effective anti-fibrotic therapies remains a critical medical need to improve the management of intestinal strictures and reduce the need for invasive procedures in Crohn's disease.
Japanese Phase 1 Study for Global Development of Anti-TL1A Antibody PF-06480605: A Randomized, Placebo-Controlled, Single-Ascending Dose StudyFukuhara, Neelakantan, Furihata
et alClin Transl Sci (2025) 18 (3), e70187
Abstract: PF-06480605, a fully human IgG1 monoclonal antibody targeting tumor necrosis factor α-like ligand 1A (TL1A), has demonstrated acceptable safety and the potential as an effective treatment for inflammatory bowel disease in phase 1/2a studies. To facilitate future clinical development in Japan and China, a Japan local phase 1 study was designed in consultation with the Japan regulatory authority. In addition to fulfilling Japan regulatory requirements, this study will bring operational efficiency and speed to global and China development by evaluating PF-06480605 in Japanese healthy adults prior to a China local phase 1 study as required by the China regulatory authority. This phase 1, randomized, double-blind, placebo-controlled, single-dose escalating study investigated the safety, tolerability, immunogenicity, pharmacokinetics (PK), and pharmacodynamics of PF-06480605 in Japanese healthy adults assigned to receive a single subcutaneous (SC) dose of PF-06480605 150 mg (N = 6), 450 mg (first-in-human dose level, N = 6), or placebo (N = 4). PF-06480605 was well tolerated and absorbed slowly with a median Tmax of 217.5 h for both 150 and 450 mg doses. Mean t1/2 was 18.4 and 19.1 days for 150 and 450 mg, respectively. Exposure parameters showed dose proportionality. No ethnic differences in PF-06480605 PK were observed. Serum TL1A levels increased in a dose-dependent manner. Immunogenicity was high with 100% of anti-PF-06480605 antibody formulation. This study satisfied the Japan regulatory requirements, while the favorable tolerability and PK of 450 mg SC in Japanese contributed to a waiver of the 150 mg SC cohort in the China local phase 1 study. NCT04269538.© 2025 The Author(s). Clinical and Translational Science published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
ELAVL1 facilitates gastric cancer progression and metastasis through TL1A mRNA stabilizationJiang, Bo, You
et alExp Cell Res (2025) 446 (2), 114491
Abstract: ELAV-like RNA-binding protein 1 (ELAVL1) is a key RNA-binding protein involved in tumor progression and metastasis. This study identifies a previously unrecognized interaction between ELAVL1 and TL1A mRNA, elucidating its role in promoting gastric cancer (GC) progression through the activation of the PI3K/Akt signaling pathway. Overexpression of ELAVL1 significantly enhances the proliferation and migration of GC cells, whereas silencing ELAVL1 leads to a marked reduction in these processes. Additionally, stable knockout of ELAVL1 significantly inhibits the growth of xenograft tumors derived from GC cells in nude mice. Mechanistically, ELAVL1 directly binds to TL1A mRNA through its RNA recognition motifs (RRM1 and RRM3). The binding sites on TL1A mRNA have been confirmed in two regions: one located between nucleotides 1605 and 1868, and the other between 4324 and 4587. ELAVL1 stabilizes TL1A mRNA expression and promotes GC progression by activating the downstream PI3K/Akt signaling pathway.Our findings highlight a novel regulatory axis involving ELAVL1, TL1A mRNA, and PI3K/Akt, providing new insights into RNA-mediated oncogenic signaling and establishing ELAVL1 as a potential therapeutic target for GC. This discovery lays the groundwork for developing targeted therapies against ELAVL1.Copyright © 2025 Elsevier Inc. All rights reserved.
TL1A, a novel alarmin in airway, intestinal, and autoimmune disordersVarricchi, Poto, Criscuolo
et alJ Allergy Clin Immunol (2025)
Abstract: The term alarmin denotes a broad class of molecules rapidly released to alert the immune system through the engagement of specific receptors on immune cells. Three alarmin cytokines-thymic stromal lymphopoietin, IL-33, and IL-25-are released from epithelial and certain stromal cells. TNF-like cytokine 1A (TL1A) is a member of the TNF cytokine superfamily, first identified in human endothelial cells. TL1A is now considered a novel alarmin expressed by human and mouse bronchial and intestinal epithelial cells. TL1A exerts its biological activities by binding to a trimeric receptor DR3 (death receptor 3), expressed on a wide spectrum of immune and structural cells, including lung fibroblasts, endothelial cells, and bronchial epithelial cells. TL1A has been implicated in experimental and human inflammatory bowel diseases as well as in airway inflammation and remodeling in severe asthma. A monoclonal antibody anti-TL1A (tulisokibart) is effective in inducing clinical remission in ulcerative colitis patients. Increasing evidence suggests that TL1A is also involved in certain autoimmune disorders, such as rheumatoid arthritis and psoriasis. These emerging findings broaden the role of TL1A in various human inflammatory conditions. Several clinical trials are currently evaluating the safety and efficacy of monoclonal antibodies targeting TL1A in asthma or inflammatory bowel disease patients.Copyright © 2025 The Authors. Published by Elsevier Inc. All rights reserved.

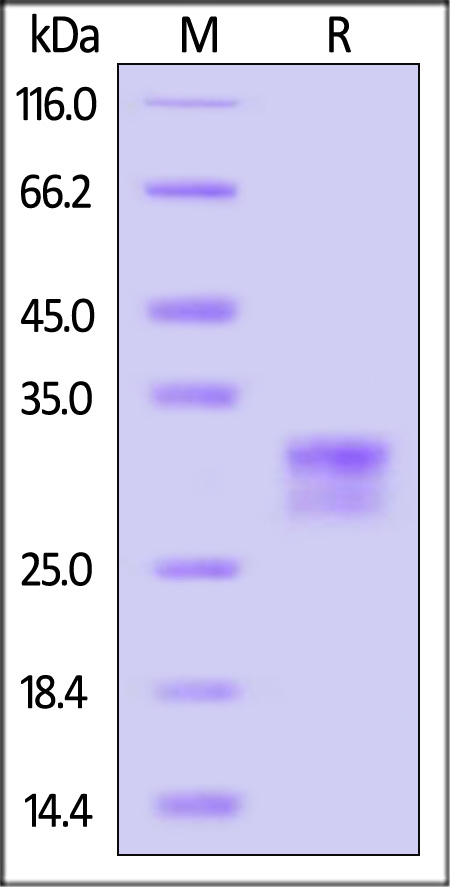
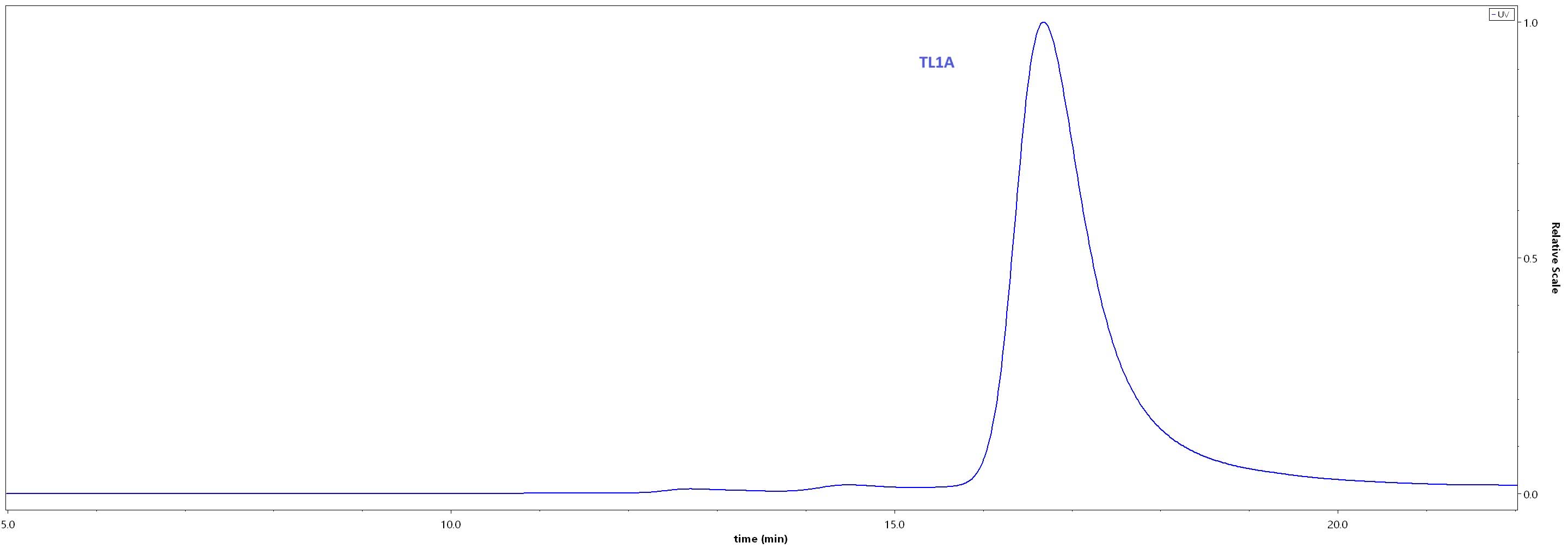
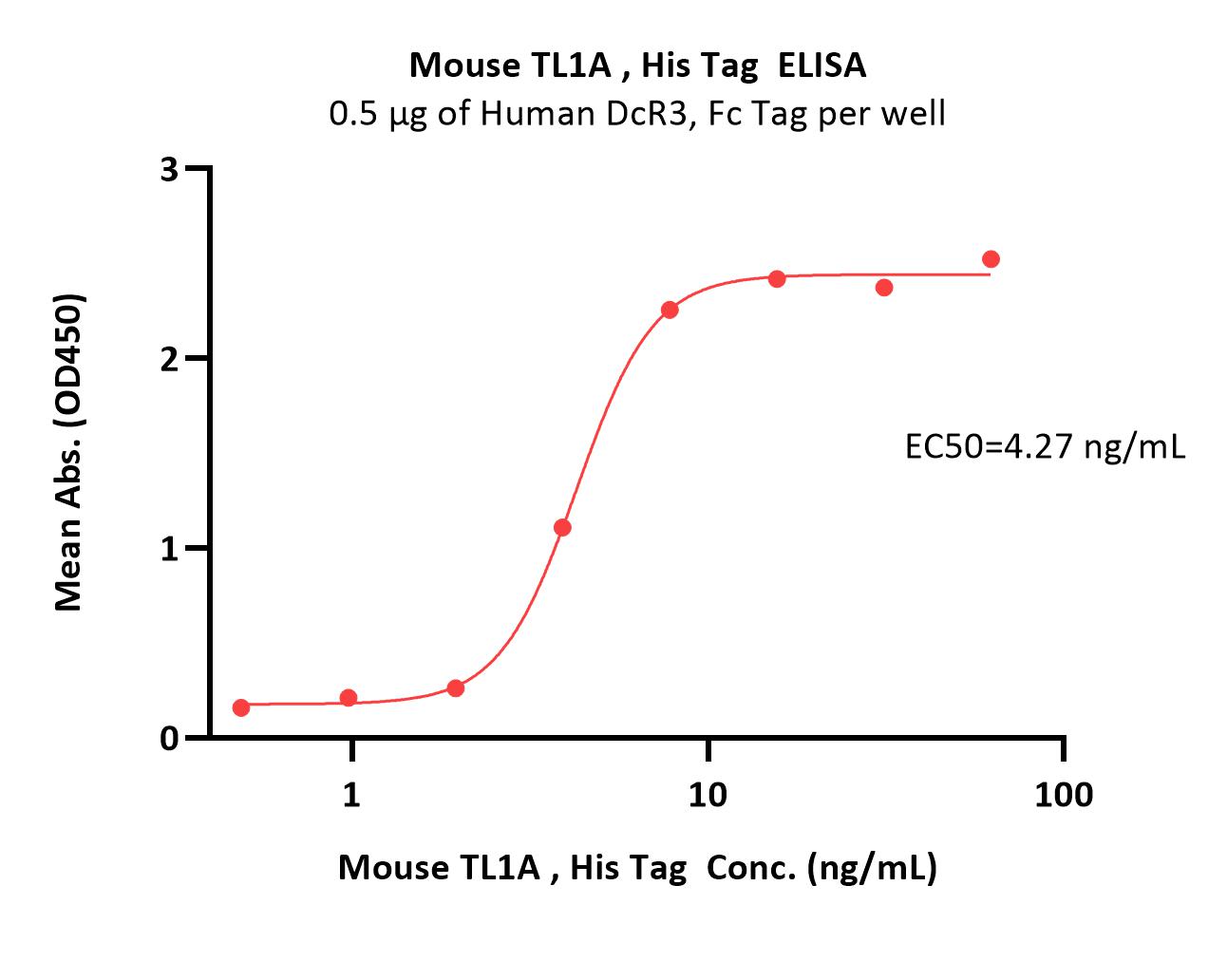

 +添加评论
+添加评论























































 膜杰作
膜杰作 Star Staining
Star Staining

















