产品概述(Product Details)
ClinMax™ Human Erythropoietin (EPO) ELISA Kit, PRO is a ready-to-use immunoassay kit, specifically designed to quantitate natural and recombinant human EPO that is present in biological samples, such as human serum, plasma, and cell culture supernatants. Our ClinMax™ ELISA Kit provides several benefits:
- Standards to calibrate with NIBSC/WHO standards for comparable results.
- Fully validation in biologic samples for detection range, sensitivity, inter- and intra-plate CV, recovery, dilution linearity, specificity, and matrix effects to ensure reliable results according to ICH M10 guideline.
- High-quality antibody pairs and protein standards, along with rigorous quality control, to guarantee consistent results across different batches.
- Simplified and straightforward protocols and ready-to-use reagents to save assay time.
应用说明(Application)
The kit is developed for quantitative detection of natural and recombinant human EPO in serum, plasma and cell culture supernatants.
It is for research use only.
流程图(Workflow)
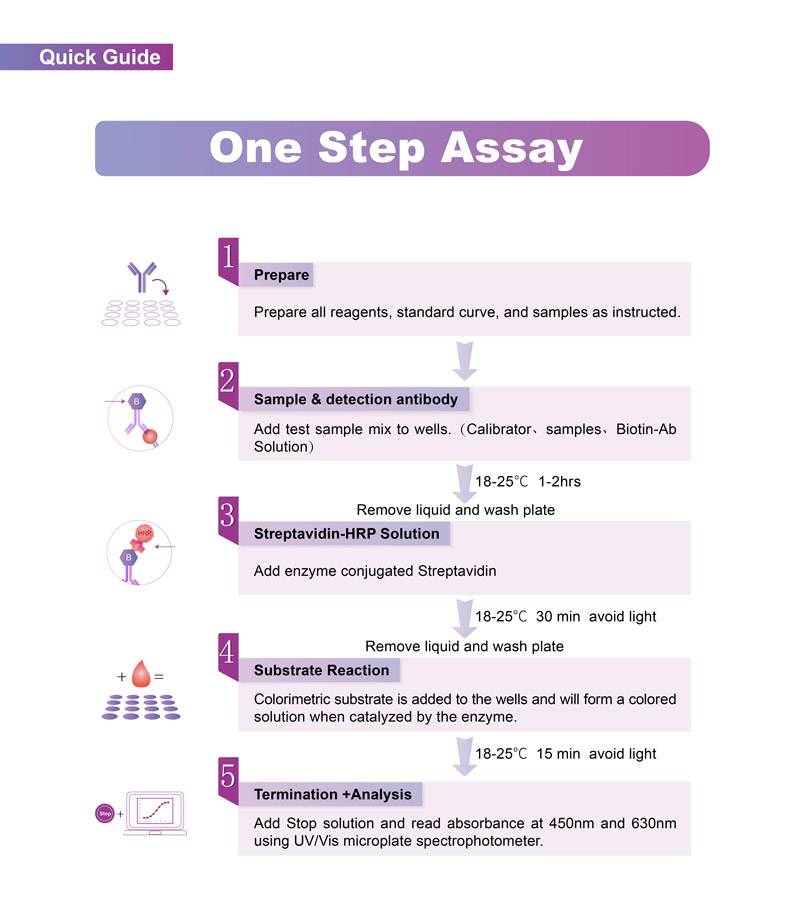
关键信息(Key Features)
| Assay Type | Sandwich-ELISA |
| Analyte | EPO |
| Format | 96-wells plate breakable into 12 x 8 wells strips |
| Reactivity | Human |
| Sensitivity | 0.67 mlU/mL |
| Assay Time | 2 hr 45 min |
| Sample volume | 50 uL |
| Range | 0.87 mlU/mL-212 mlU/mL |
| Sample Type | Cell Culture Supernatants, Plasma, Serum. |
| NIBSC Code | 11/170 |
Elevate your research experience with our Cytokine/Biomarker Detection Kits, where accuracy, reliability, and ease of use are converging to deliver exceptional results.
存储(Storage)
Keep the unopened kit stored at 2-8 °C. Avoid using the kit beyond its expiration date. The opened kit should be stored per components table. The shelf life is 30 days from the date of opening.
典型数据-Typical Data Please refer to DS document for the assay protocol.
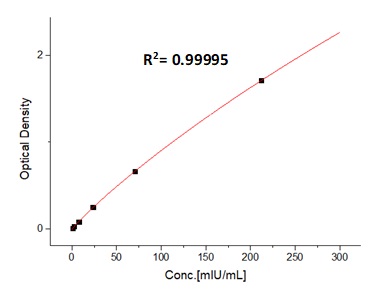
For each experiment, each ELISA plate needs to set the standard curve. The minimum detectable concentration of EPO is less than 0.67 mIU/mL.
验证(Validation)
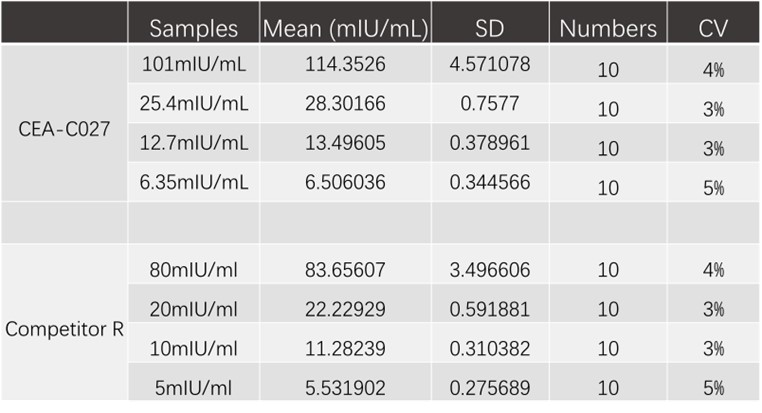
精密度(Precision)
To evaluate the intra-assay precision of assay using CEA-C027 and Competitor R, four samples spiked with different concentrations of EPO were tested. Results shown in the following Table. CV values of all analytes are less than 6%. Acceptable criteria : CV<10%.
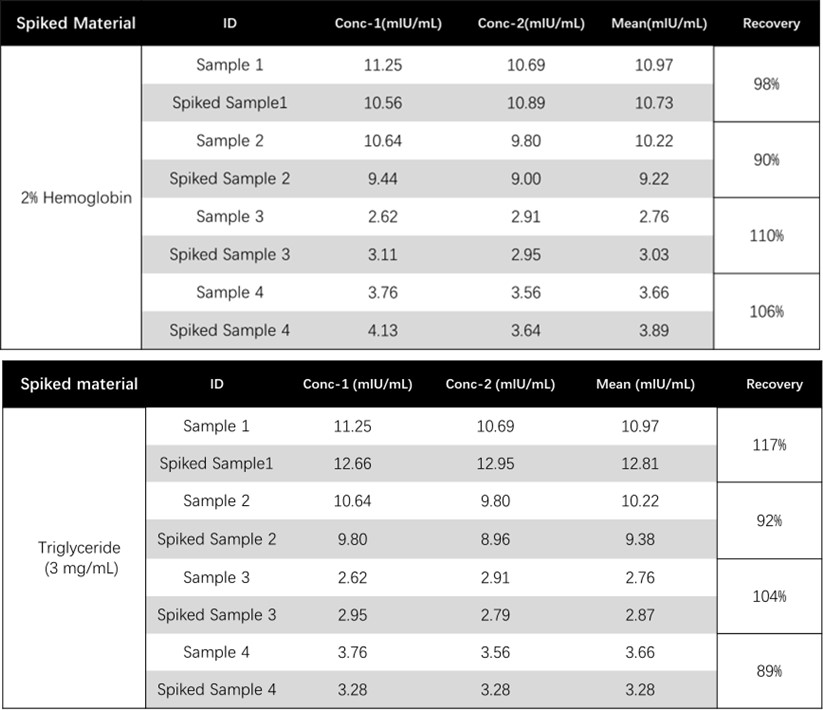
基质效应(Matrix Effect)
To evaluate the hemolysis matrix effect and high-dose triglyceride matrix effect of assay, serum samples spiked with high concentrations of hemoglobin (2%), or triglyceride (3 mg/mL) were tested. Results shown that all spiked analytes had recoveries between 90% and 110%, no hemolysis matrix effect and high-dose triglyceride matrix effect was observed in assay using CEA-C027.
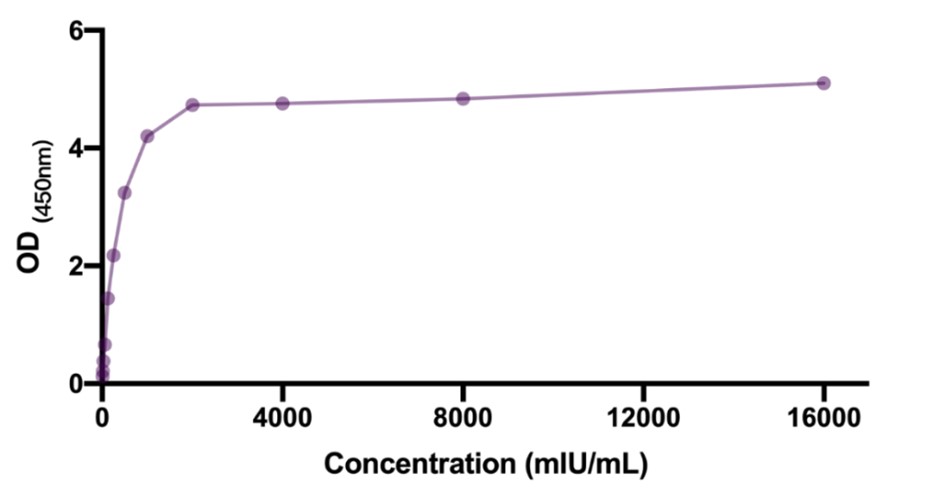
钩状效应(Hook Effect)
To evaluate the hook effect of the assay, some samples with high concentration of EPO were tested. Results shown in the following figure. No hook effect was found in the assay using the CEA-C027.
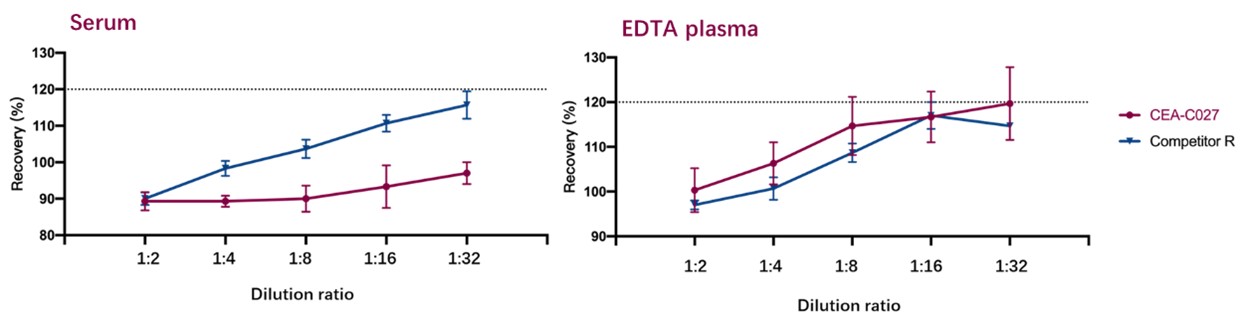
稀释线性(Dilution Linearity)
To evaluate the linearity of assay using CEA-C027 and Competitor R, samples (Serum, EDTA plasma) spiked with high concentrations of EPO were serially diluted with dilution buffer to produce samples with values within the dynamic range of the assay. Results shown that recoveries of all analytes are less than 120%. Acceptable criteria: Recovery should be between 80% and 120%.
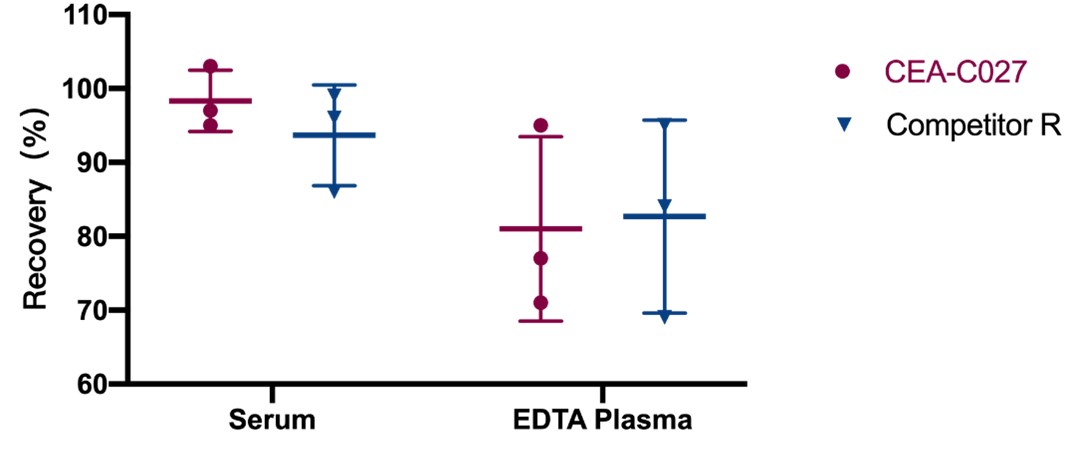
回收率(Recovery)
To evaluate the recovery of assay using CEA-C027 and Competitor R, samples (Serum, EDTA plasma) spiked with low concentrations of EPO (LQC) were tested. All analytes had recoveries between 80% and 100%. Acceptable criteria: Recovery should be between 80% and 120%.
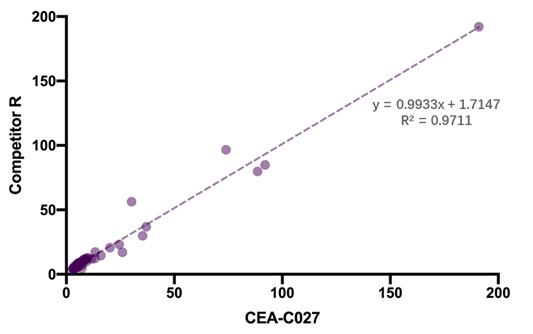
一致性(Consistency)
To evaluate the consistency of assay using CEA-C027 and Competitor R, 40 healthy serum samples and 14 patient serum samples were tested, and these results demonstrated a high degree of consistency between the two products, R² >0.95.
组分(Materials Provided)
| ID | Components | Size |
| CEA027-C01 | Pre-coated Anti-EPO Antibody Microplate | 1 plate |
| CEA027-C02 | Human EPO Standard | 3392 IU×2 |
| CEA027-C03 | Biotin-Anti-EPO Antibody Con. Solution | 100 μL |
| CEA027-C04 | Biotin-Antibody Dilution Buffer | 8 mL |
| CEA027-C05 | Streptavidin-HRP Con. Solution | 500 μL |
| CEA027-C06 | HRP Dilution Buffer | 15 mL |
| CEA027-C07 | 20× Washing Buffer | 50 mL |
| CEA027-C08 | Sample Dilution Buffer | 15 mL×2 |
| CEA027-C09 | Substrate Solution | 12 mL |
| CEA027-C10 | Stop Solution | 6 mL |























































 膜杰作
膜杰作 Star Staining
Star Staining












