背景(Background)
Residual host cell DNA refers to fragments of DNA from the host organism that are left behind after a biological process such as the production of a biopharmaceutical product or the cultivation of a cell line. This residual DNA can potentially contaminate the final product and affect its safety and efficacy. In the context of biopharmaceutical production, regulatory agencies such as the FDA and EMA have set limits on the amount of residual host cell DNA that is acceptable in a final product. These limits vary depending on the type of product and the route of administration, and residue host cell DNA quantitative kits are designed to ensure that the final product is safe for human use.
产品描述(Product Details)
CHO resDNA Quantitation Kit is designed for quantitative detection of residual CHO DNA in biopharmaceuticals (Antibodies). Based on real-time quantitative PCR (qPCR) method, this kit this kit makes the detection of the residual DNA from CHO cells rapid and reliable. The lower limit of quantitation is 3 fg/µL. All procedures are typically in less than 4 hours.
Use this kit to detect residual DNA after the DNA is extracted from test samples. For achieving the better DNA recovery, it is recommended to use the resDetect™ resDNA Sample Preparation Kit (Magnetic Beads) (Cat. No. OPA-R005) in combination.
产品特性(Features)
- High sensitivity for optimal product safety: LLOD (1 fg/µL) for detection of residue host cell DNA
- High specificity: No cross-reactivity with unrelated DNA
- Validation: ICH Q2(R2) as validation of analytical procedures
- High-quality: This kit is manufactured in GMP facility and alignment with the ISO 13485 standard.
应用说明(Application)
The kit is used for quantitative detection of residual CHO DNA in biopharmaceutical production.
For use in quality control/manufacturing process only.
It is for research use only.
技术参数(Technical Specifications)

使用提示(Attention)
If your experimental process requires sample preparation, please purchase and conduct the experiment using the sample preparation kits recommended in the table to ensure that the buffers used in both the sample preparation and resDNA detection are consistent.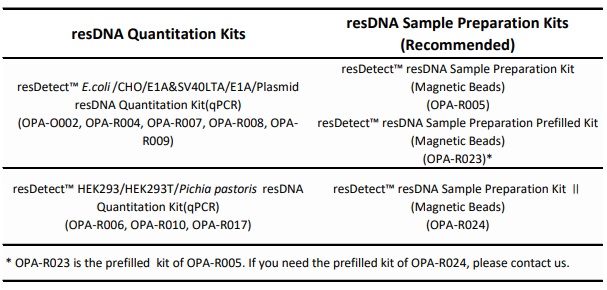
典型数据-Typical Data Please refer to DS document for the assay protocol.
High sensitive: LLOQ (Lower limit of quantitation) is 3 fg/μL
Figure 1. High sensitivity and broad dynamic range using the CHO resDNA Quantitation Kit. (A) Typical analysis results obtained with Standard 1 (300 pg/μL) to 6 (3 fg/μL). (B) The standard curve of the 10-fold dilution series. PCR efficiency should be 90-110%.

High specificity: No cross-reactivity with unrelated DNA
Figure 2. Assay specificity. Standard curves generated using 10-fold serial dilution of CHO genomic DNA (included in the kit).

High consistency:Consistent performance across the expected range of DNA fragment sizes
Figure 3. Consistent quantitation across a broad range of fragment sizes. Standard curves were generated using a 10-fold serial dilution of gDNA and fragmented DNA. Fragmented DNA was generated by sonicating total CHO genomic DNA (10 min, 20 min, 30min).

The validation of standard curve
Figure 4. The amplification efficiency of standard curve (ACRO and Competitor J) is close to 100%, while the amplification efficiency of standard curve (Competitor H) is less than 90%.

The validation of spike recovery
Figure 5. The validation of spike recovery in three spike levels CHO DNA samples (high, medium, low). Results show that the recovery using the ACRO products are between 90 and 100%.

Complex Sample Simulation Testing
For samples with high concentration BSA (up to 150 mg/mL) and different pH buffers (3.0 ≤ pH ≤ 10.0), the resDetectTM kits were used for the extraction and detection of CHO resDNA. The acceptable spiked recovery range should be 50-150%. The results showed that the spiked recoveries were all between 98-120%. The results are shown in the table below.
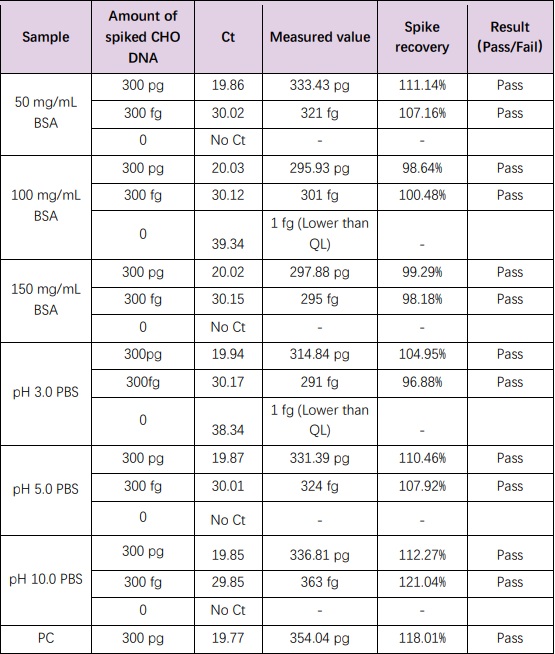
Testing of complex samples from CHO antibody purification process
For samples from different steps (AF, VI, AEX, and DF) in the CHO antibody purification process development , the extraction and detection of CHO resDNA were performed using the resDetectTM kits. The acceptable spiked recovery range is 50-150%. The results showed that the spiked recoveries for the various intermediate samples were all close to 100%.
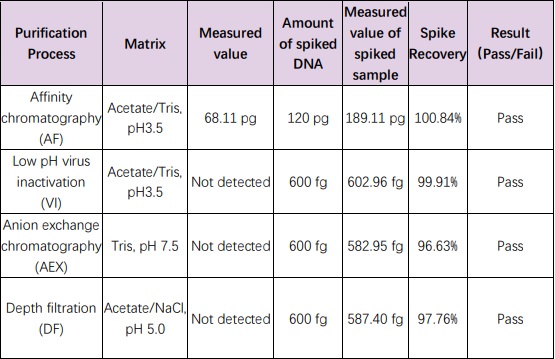
组分(Materials Provided)
| ID | Components | Size |
| OPA-R004-01 | 2×qPCR Master Mix | 1.0 mL×2 |
| OPA-R004-02 | CHO Primer & Probe Mix | 700 μL |
| OPA-R004-03 | CHO DNA Control(3ng/μL) | 100 μL |
| OPA-R004-04 | Dilution Buffer | 1.5 mL×3 |
| OPA-R004-05 | DNase/RNase-Free Water | 1.0 mL |























































 膜杰作
膜杰作 Star Staining
Star Staining











