分子别名(Synonym)
capsid protein
表达区间及表达系统(Source)
AAV2 VP1, Recombinant Protein (VP1-A5143) is expressed from E. coli cells. It contains AA Met1 - Leu735 (Accession # P03135-1).
Predicted N-terminus: Met
Request for sequence
蛋白结构(Molecular Characterization)

This protein carries a polyhistidine tag at the N-terminus.
The protein has a calculated MW of 84.0 kDa. The protein migrates as 45-90 kDa when calibrated against Star Ribbon Pre-stained Protein Marker under reducing (R) condition (SDS-PAGE).
内毒素(Endotoxin)
Less than 1.0 EU per μg by the LAL method.
纯度(Purity)
>85% as determined by SDS-PAGE.
制剂(Formulation)
Supplied as 0.2 μm filtered solution in 20 mM Tris, 0.5 M NaCl, 0.5 M Arginine, pH8.0 with trehalose as protectant.
Contact us for customized product form or formulation.
运输(Shipping)
This product is supplied and shipped with dry ice, please inquire the shipping cost.
存储(Storage)
Please avoid repeated freeze-thaw cycles.
This product is stable after storage at:
- The product MUST be stored at -70°C or lower upon receipt;
- -70°C for 3 months under sterile conditions.
电泳(SDS-PAGE)
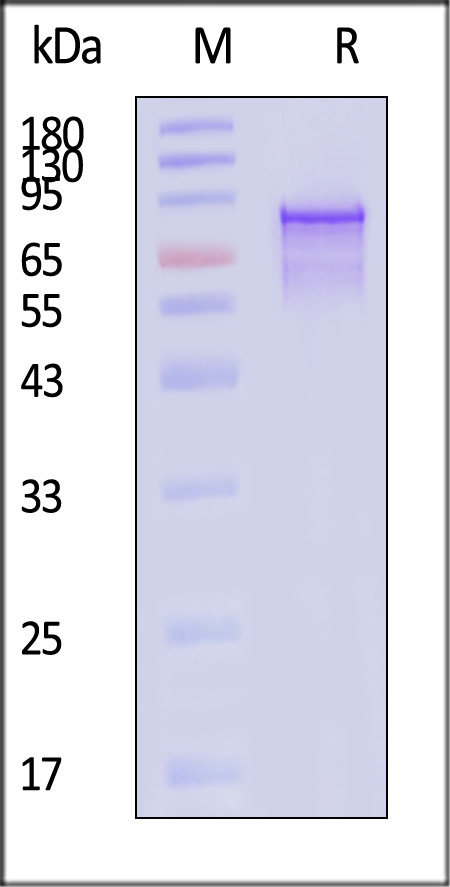
AAV2 VP1, Recombinant Protein on SDS-PAGE under reducing (R) condition. The gel was stained with Coomassie Blue. The purity of the protein is greater than 85% (With Star Ribbon Pre-stained Protein Marker).
活性(Bioactivity)-ELISA
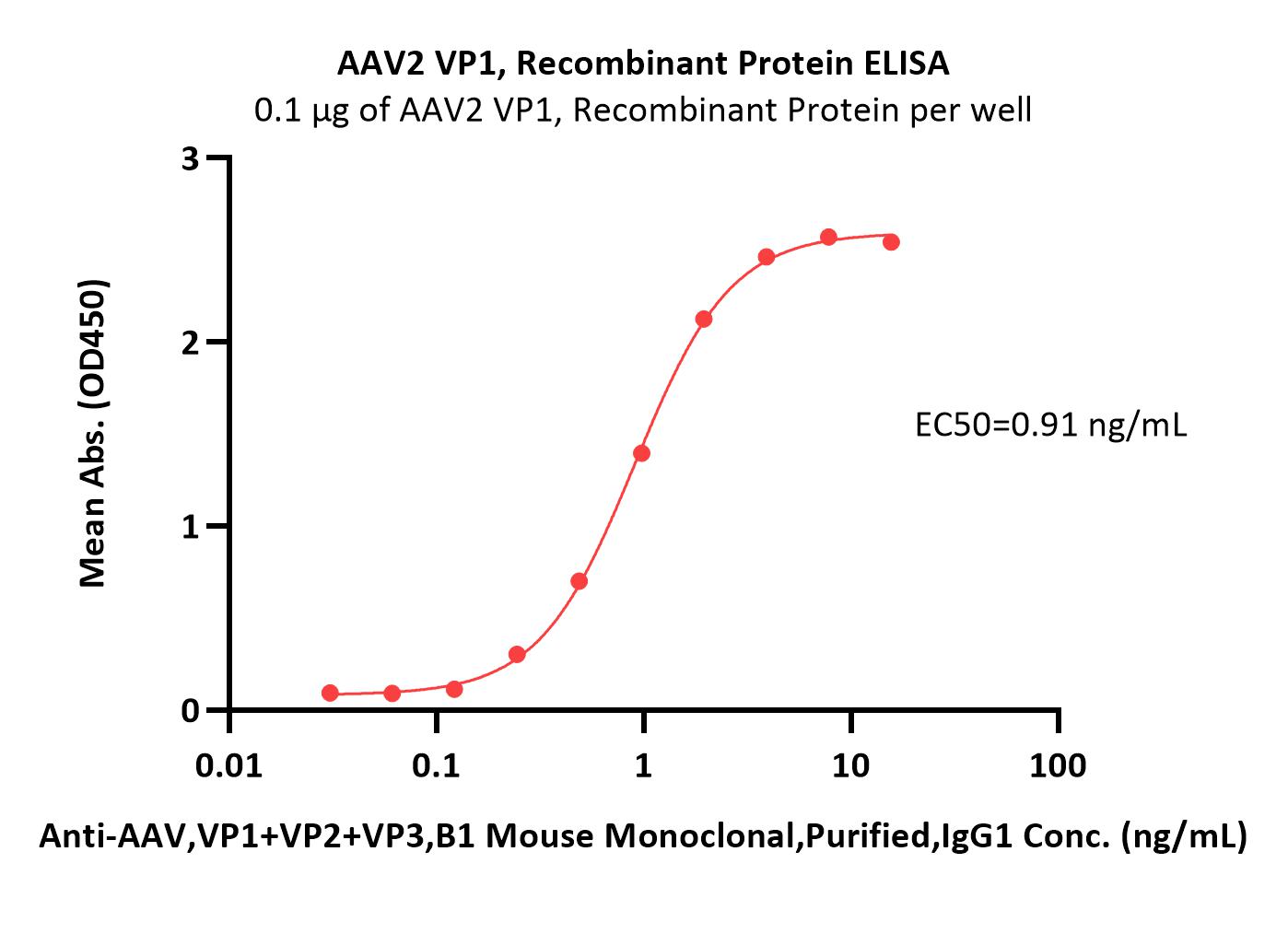
Immobilized AAV2 VP1, Recombinant Protein (Cat. No. VP1-A5143) at 1 μg/mL (100 μL/well) can bind Anti-AAV,VP1+VP2+VP3,B1 Mouse Monoclonal,Purified,IgG1 with a linear range of 1-31 ng/mL (QC tested).
Protocol
背景(Background)
Adeno-associated virus is a single-stranded DNA virus and the current scientific consensus is that it does not cause any human disease. It consists of a protein capsid (CAPside) and a 4.7 KB length single stranded DNA genome. The protein capsid consists of three subunits, VP1, VP2, and VP3.























































 膜杰作
膜杰作 Star Staining
Star Staining











