Integrated virtual screening and compound generation targeting H275Y mutation in the neuraminidase gene of oseltamivir-resistant influenza strainsKhan, Khan, Tembhre
et alMol Divers (2025)
Abstract: Neuraminidase (NA) is an essential enzyme located at the outer layer of the influenza virus and plays a key role in the release of virions from infected cells. The rising incidence of global epidemics has made the urgent need for effective antiviral medications an urgent public health priority. Furthermore, the emergence of resistance caused by specific mutations in the influenza viral genome exacerbates the challenges of antiviral therapy. In view of this, this study aims to identify and analyse possible inhibitors of NA from different subtypes of influenza viruses. Initially, a thorough search was conducted in the Protein Data Bank (PDB) to gather structures of NA proteins that were attached with oseltamivir, a widely recognized inhibitor of NA. Here, 36 PDB entries were found with NA-oseltamivir complexes which were studied to evaluate the diversity and mutations present in various subtypes. Finally, N1(H1N1) protein was selected that demonstrated low IC50 value of oseltamivir with mutation H275Y. In addition, the study utilized BiMODAL generative model to generate 1000 novel molecules with comparable structures to oseltamivir. A QSAR model, based on machine learning (ML), was built utilizing the ChEMBL database to improve the selection process of candidate inhibitors. These inhibitors were subsequently analysed by molecular docking and further the best hits compounds (compound_375, compound_106 and compound_597) were appended to make a bigger molecule (compound_106-375, compound_106-597, and compound_375-597) to fit into the binding pocket of protein. Further, triplicate molecular dynamics simulations lasting 100 ns to assess their effectiveness and binding stability showed that compound_106-375 had the most stable binding with the protein. Key residues, including Asn146, Ala138, and Tyr155, form critical interactions with the ligand, contributing to its stability. The investigation was enhanced by employing principal component analysis (PCA), free energy landscape (FEL), and binding free energy calculations. The total binding free energy (GTOTAL) of - 169.62 kcal/mol suggests that the contact between compound_106-375 and the mutant N1 (H1N1) protein is thermodynamically favourable. This approach allowed for a thorough comprehension of the binding interactions and possible effectiveness of the discovered inhibitors. Overall, these findings demonstrate that compound_106-375 exhibits favourable binding characteristics and stability. Further experimental validation is required to confirm its efficacy against the H275Y mutant neuraminidase protein and its potential to overcome influenza drug resistance. However, compound_106-375 is suggested as a promising candidate for further development as a therapeutic agent against the mutant N1 (H1N1) protein. This finding will assist in drug development and to overcome the challenges associated with drug resistance in influenza strains.© 2025. The Author(s), under exclusive licence to Springer Nature Switzerland AG.
Antigenicity and genetic properties of an Eurasian avian-like H1N1 swine influenza virus in Jiangsu Province, ChinaHe, Yu, Liu
et alBiosaf Health (2024) 6 (6), 319-326
Abstract: Pigs are vital genetic mixing vessels for human and avian influenza viruses because their tracheal epitheliums possess both sialic acid α-2,6-Gal and α-2,3-Gal receptors. Cross-species transmission of influenza A viruses from swine to humans occurs occasionally. The first case of human infection with the Eurasian avian-like H1N1 swine influenza virus (EAH1N1 SIVs) genotype G4 was detected in Jiangsu Province, China, in February 2023, and backtracking epidemiological investigations did not reveal a clear source of the infection. The hemagglutination (HA) and neuraminidase (NA) amino acid variant sites, antiviral drug susceptibility, and antigenic variation of the isolated A/Jiangsu/27271/2023 (JS/27271/23) virus were analyzed, and we evaluated the protective effect of sera collected from occupationally exposed populations in 2024 against the virus. Compared with the vaccine strain, the nucleotide sequence similarities of JS/27271/23 HA and NA were 96.5 % and 95.2 %, respectively. JS/27271/23 was sensitive to polymerase inhibitors (favipiravir and baloxavir), and the antigenicity of its HA protein was 8-fold different from that of the vaccine strain. The percentage of occupationally exposed population with antibody titers of ≥ 40 against A/Hunan/42443/2015 (HN/42443/15) and A/Jiangsu/1/2011 (JS/1/11) were 7.25 % and 2.25 %, respectively, and the geometric mean titers (GMT) were 6.24 and 5.34, respectively. Out of 400 serum samples examined, none had antibody titers of ≥ 40 against JS/27271/23. This suggests that low serum levels of antibodies to EAH1N1 SIVs in occupationally exposed populations may not provide adequate protection because of significant differences in amino acid sites and antigenicity between this virus and the current vaccine strain of EAH1N1 SIVs. There is no evidence of human-to-human transmission of EAH1N1 SIVs. Therefore, surveillance for EAH1N1 SIVs and the development of new vaccine strains are required.© 2024 Chinese Medical Association Publishing House. Published by Elsevier B.V.
A replicating recombinant vesicular stomatitis virus model for dairy cattle H5N1 influenza virus glycoprotein evolutionRobinson-McCarthy, Zirckel, Simmons
et albioRxiv (2025)
Abstract: A panzootic of highly pathogenic avian influenza (HPAI) H5N1 viruses from clade 2.3.4.4b has triggered a multistate outbreak in United States dairy cattle and an unknown number of human infections. HPAI viruses are handled in specialized biocontainment facilities. Ethical considerations limit certain experimental evolution experiments aimed at assessing viral resistance to potential therapeutics. We have developed a replicating recombinant vesicular stomatitis virus (rVSV) where we replaced its glycoprotein with the hemagglutinin (HA) and neuraminidase (NA) genes of a 2.3.4.4b H5N1 virus (rVSV-H5N1dc2024), which is free of these constraints. This virus grows to high titers and encodes a fluorescent reporter to track infection. We demonstrate the utility of rVSV-H5N1dc2024 in neutralization experiments, evaluating antibody escape and characterization of resistance mutations to NA inhibitors. rVSV-H5N1dc2024 or similar viruses may accelerate efforts to develop and evaluate interventions against this emerging threat to human and animal health.
Influenza Neuraminidase Virus-Like Particle-Based Nanocarriers as a New Platform for the Delivery of Small-Peptide AntigensKhanefard, Trianti, Akeprathumchai
et alMol Biotechnol (2025)
Abstract: A new and simple platform to produce a nanocarrier for small-peptide antigen delivery was developed. Virus-like particles (VLPs) were of interest due to their good cell-penetrating properties and ability to protect target molecules from degradation. In this study, the VLP that was entirely formed by influenza neuraminidase (NA), NA-VLPs, was employed. The platform construction includes the genetic engineering of target peptides into the NA structure immediately above its stalk, at the bottom of the NA head, by an overlap extension PCR. The resulting chimeric gene is next expressed in stably transformed insect cells. The recombinant NA protein produced by the insect cells is then naturally assembled into the NA-VLPs that display those peptides on their surfaces. For the platform demonstration, Angiotensin II (AngII) octapeptide hormones that raise blood pressure were chosen as a model peptide antigen. The NA-VLPs displaying AngII peptides were successfully produced by the stably transformed insect cells. The AngII octapeptides were successfully delivered by the NA-AngII VLPs as the anti-AngII antibodies were raised in hypertensive rats. The antibodies effectively neutralized the AngII peptide hormone in these rats, as demonstrated by the decrease in systolic blood pressure of the immunized rats. Thus, NA-VLP nanocarriers represent a promising platform for delivering small-peptide antigens to stimulate antibody production.© 2025. The Author(s), under exclusive licence to Springer Science+Business Media, LLC, part of Springer Nature.

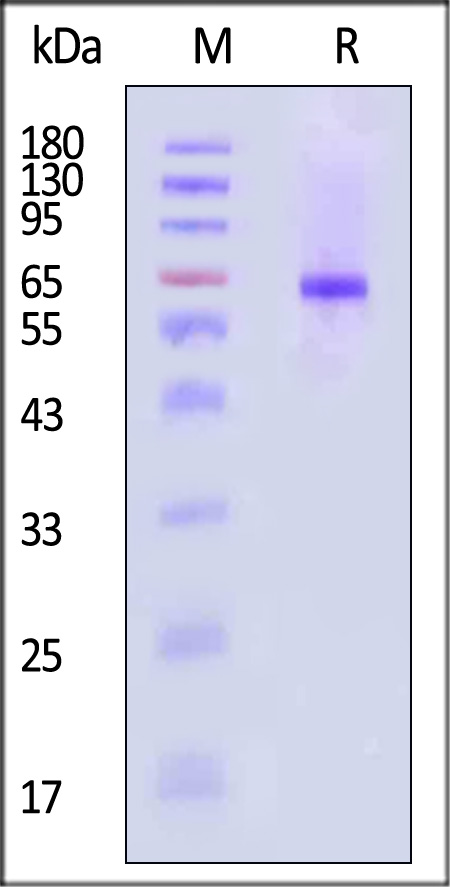























































 膜杰作
膜杰作 Star Staining
Star Staining















