FGF-17 from Hypoxic Human Wharton's Jelly-Derived Mesenchymal Stem Cells Is Responsible for Maintenance of Cell Proliferation at Late PassagesHan, Kim, Jeong
et alInt J Stem Cells (2019) 12 (2), 279-290
Abstract: Although it is well known that hypoxic culture conditions enhance proliferation of human mesenchymal stem cells, the exact mechanism is not fully understood. In this study, we investigated the effect of fibroblast growth factor (FGF)-17 from hypoxic human Wharton's Jelly-derived mesenchymal stem cells (hWJ-MSCs) on cell proliferation at late passages.hWJ-MSCs were cultured in α-MEM medium supplemented with 10% fetal bovine serum (FBS) in normoxic (21% O2) and hypoxic (1% O2) conditions. Protein antibody array was performed to analyze secretory proteins in conditioned medium from normoxic and hypoxic hWJ-MSCs at passage 10. Cell proliferation of hypoxic hWJ-MSCs was increased compared with normoxic hWJ-MSCs from passage 7 to 10, and expression of secretory FGF-17 was highly increased in conditioned medium from hypoxic hWJ-MSCs at passage 10. Knockdown of FGF-17 in hypoxic and normoxic hWJ-MSCs decreased cell proliferation, whereas treatment of hypoxic and normoxic hWJ-MSCs with recombinant protein FGF-17 increased their proliferation. Signal transduction of FGF-17 in hypoxic and normoxic hWJ-MSCs involved the ERK1/2 pathway. Cell phenotypes were not changed under either condition. Differentiation-related genes adiponectin, Runx2, and chondroadherin were downregulated in normoxic hWJ-MSCs treated with rFGF-17, and upregulated by siFGF-17. Expression of alkaline phosphatase (ALP), Runx2, and chondroadherin was upregulated in hypoxic hWJ-MSCs, and this effect was rescued by transfection with siFGF-17. Only chondroadherin was upregulated in hypoxic hWJ-MSCs with rFGF-17.In hypoxic culture conditions, FGF-17 from hypoxic hWJ-MSCs contributes to the maintenance of high proliferation at late passages through the ERK1/2 pathway.
FGF-2 deficiency does not influence FGF ligand and receptor expression during development of the nigrostriatal systemRatzka, Baron, Grothe
PLoS One (2011) 6 (8), e23564
Abstract: Secreted proteins of the fibroblast growth factor (FGF) family play important roles during development of various organ systems. A detailed knowledge of their temporal and spatial expression profiles, especially of closely related FGF family members, are essential to further identification of specific functions in distinct tissues. In the central nervous system dopaminergic neurons of the substantia nigra and their axonal projections into the striatum progressively degenerate in Parkinson's disease. In contrast, FGF-2 deficient mice display increased numbers of dopaminergic neurons. In this study, we determined the expression profiles of all 22 FGF-ligands and 10 FGF-receptor isoforms, in order to clarify, if FGF-2 deficiency leads to compensatory up-regulation of other FGFs in the nigrostriatal system. Three tissues, ventral mesencephalon (VM), striatum (STR) and as reference tissue spinal cord (SC) of wild-type and FGF-2 deficient mice at four developmental stages E14.5, P0, P28, and adult were comparatively analyzed by quantitative RT-PCR. As no differences between the genotypes were observed, a compensatory up-regulation can be excluded. Moreover, this analysis revealed that the majority of FGF-ligands (18/22) and FGF-receptors (9/10) are expressed during normal development of the nigrostriatal system and identified dynamic changes for some family members. By comparing relative expression level changes to SC reference tissue, general alterations in all 3 tissues, such as increased expression of FGF-1, -2, -22, FgfR-2c, -3c and decreased expression of FGF-13 during postnatal development were identified. Further, specific changes affecting only one tissue, such as increased FGF-16 (STR) or decreased FGF-17 (VM) expression, or two tissues, such as decreased expression of FGF-8 (VM, STR) and FGF-15 (SC, VM) were found. Moreover, 3 developmentally down-regulated FGFs (FGF-8b, FGF-15, FGF-17a) were functionally characterized by plasmid-based over-expression in dissociated E11.5 VM cell cultures, however, such a continuous exposure had no influence on the yield of dopaminergic neurons in vitro.
Thymosin beta4 mediated PKC activation is essential to initiate the embryonic coronary developmental program and epicardial progenitor cell activation in adult mice in vivoBock-Marquette, Shrivastava, Pipes
et alJ Mol Cell Cardiol (2009) 46 (5), 728-38
Abstract: Hypoxic heart disease is a predominant cause of disability and death worldwide. Since adult mammalian hearts are incapable of regeneration after hypoxia, attempts to modify this deficiency are critical. As demonstrated in zebrafish, recall of the embryonic developmental program may be the key to success. Because thymosin beta4 (TB4) is beneficial for myocardial cell survival and essential for coronary development in embryos, we hypothesized that it reactivates the embryonic developmental program and initiates epicardial progenitor mobilization in adult mammals. We found that TB4 stimulates capillary-like tube formation of adult coronary endothelial cells and increases embryonic endothelial cell migration and proliferation in vitro. The increase of blood vessel/epicardial substance (Bves) expressing cells accompanied by elevated VEGF, Flk-1, TGF-beta, Fgfr-2, Fgfr-4, Fgf-17 and beta-Catenin expression and increase of Tbx-18 and Wt-1 positive myocardial progenitors suggested organ-wide recall of the embryonic program in the adult epicardium. TB4 also positively regulated the expression and phosphorylation of myristoylated alanine-rich C-kinase substrate (Marcks), a direct substrate and indicator of protein kinase C (PKC) activity in vitro and in vivo. PKC inhibition significantly reduced TB4 initiated epicardial thickening, capillary growth and the number of myocardial progenitors. Our results demonstrate that TB4 is the first known molecule capable of organ-wide activation of the embryonic coronary developmental program in the adult mammalian heart after systemic administration and that PKC plays a significant role in the process.
Fibroblast growth factor receptor 4 predicts failure on tamoxifen therapy in patients with recurrent breast cancerMeijer, Sieuwerts, Look
et alEndocr Relat Cancer (2008) 15 (1), 101-11
Abstract: Tamoxifen treatment of estrogen-dependent breast cancer ultimately loses its effectiveness due to the development of resistance. From a functional screen for identifying genes responsible for tamoxifen resistance in human ZR-75-1 breast cancer cells, fibroblast growth factor (FGF) 17 was recovered. The aim of this exploratory study was to assess the predictive value of FGF17 and the receptors FGFR1-4 for the type of response to tamoxifen treatment (clinical benefit) and the duration of progression-free survival (PFS) in patients with recurrent breast cancer. mRNA levels of FGF17 and FGFR1-4 were quantified by real-time reverse transcriptase PCR in 285 estrogen receptor-positive breast carcinomas with clinical follow-up. All patients had recurrent disease and were treated with tamoxifen as first-line systemic therapy for local or distant relapse. FGF17 and FGFR1-3 mRNA levels had no significant predictive value for this group of patients. However, high FGFR4 mRNA levels analyzed as a continuous log-transformed variable predicted poor clinical benefit (odds ratio=1.22; P=0.009) and shorter PFS (hazard ratio=1.18; P<0.001). In addition, in multivariable analysis, the predictive value of FGFR4 was independent from the traditional predictive factors. Our analyses show that FGFR4 may play a role in the biological response of the tumor to tamoxifen treatment. In addition, as altered expression of FGF17 causes tamoxifen resistance in vitro, the FGF signaling pathway could be a valuable target in the treatment of breast cancer patients resistant to endocrine treatment.




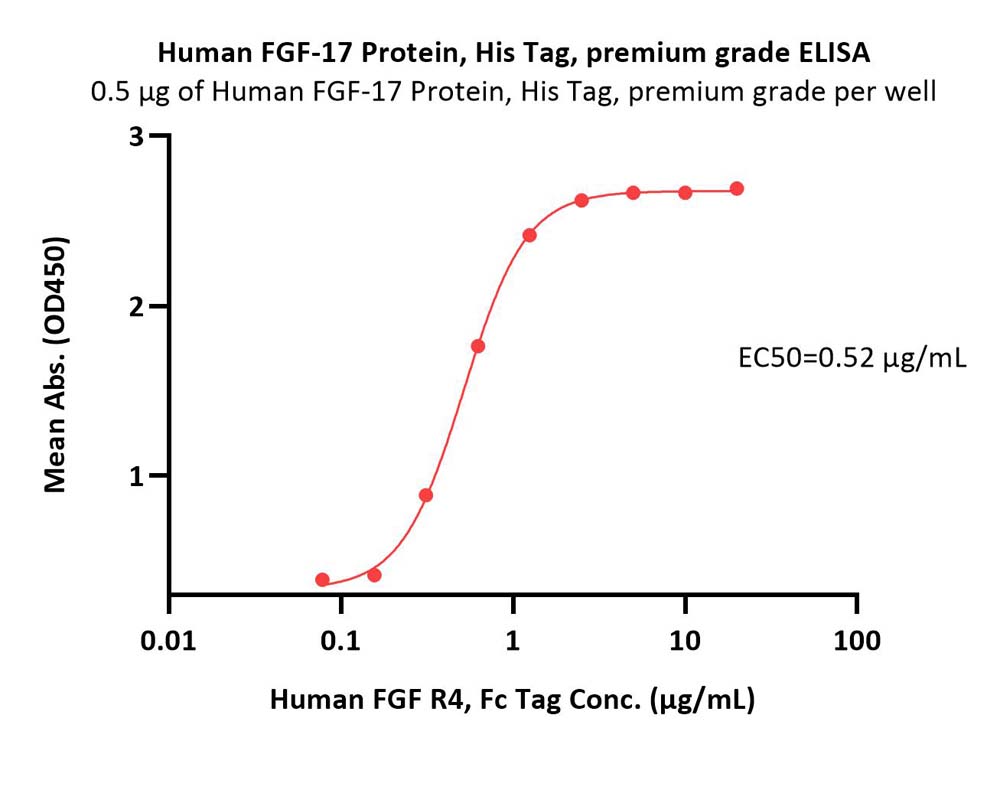
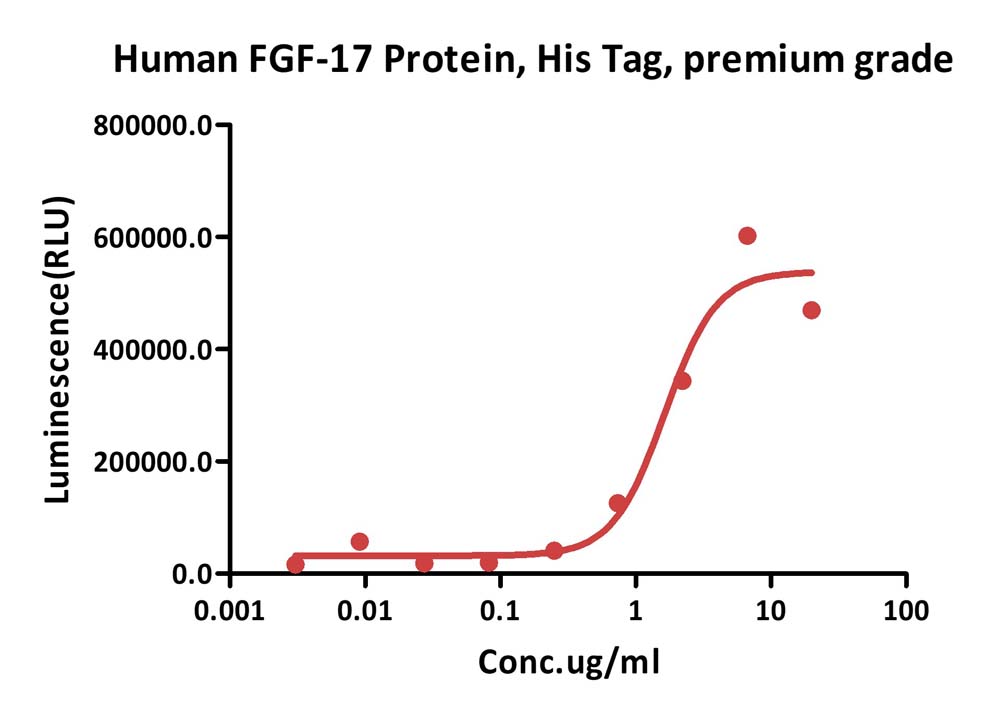























































 膜杰作
膜杰作 Star Staining
Star Staining















