First-in-human phase I trial of the bispecific CD47 inhibitor and CD40 agonist Fc-fusion protein, SL-172154 in patients with platinum-resistant ovarian cancerLakhani, Stewart, Richardson
et alJ Immunother Cancer (2025) 13 (1)
Abstract: SL-172154 is a hexameric fusion protein adjoining the extracellular domain of SIRPα to the extracellular domain of CD40L via an inert IgG4-derived Fc domain. In preclinical studies, a murine equivalent SIRPα-Fc-CD40L fusion protein provided superior antitumor immunity in comparison to CD47- and CD40-targeted antibodies. A first-in-human phase I trial of SL-172154 was conducted in patients with platinum-resistant ovarian cancer.SL-172154 was administered intravenously at 0.1, 0.3, 1.0, 3.0, and 10.0 mg/kg. Dose escalation followed a modified toxicity probability interval-2 design. Objectives included evaluation of safety, dose-limiting toxicity, recommended phase II dose, pharmacokinetic (PK) and pharmacodynamic (PD) parameters, and antitumor activity.27 patients (median age 66 years (range, 33-85); median of 4 prior systemic therapies (range, 2-9)) with ovarian (70%), fallopian tube (15%), or primary peritoneal (15%) cancer received SL-172154. Treatment-emergent adverse events (TEAEs) were reported for 27 patients (100%), with 24 (88.9%) having a drug-related TEAE and infusion-related reactions being the most common. 12 patients (44.4%) had grade 3/4 TEAEs, and half of these patients (22.2%) had a drug-related grade 3/4 TEAE. There were no fatal adverse events, and no TEAEs led to drug discontinuation. SL-172154 Cmax and area under the curve increased with dose with greater than proportional exposure noted at 3.0 and 10.0 mg/kg. CD47 and CD40 target engagement on CD4+ T cells and B cells, respectively, approached 100% by 3.0 mg/kg. Dose-dependent responses in multiple cytokines (eg, interleukin 12 (IL-12), IP-10) approached a plateau at ≥3.0 mg/kg. Paired tumor biopsies demonstrated a shift in macrophages from an M2- to an M1-dominant phenotype and increased infiltration of CD8 T cells. PK/PD modeling showed near maximal margination of B cells and a dose-dependent production of IL-12 nearing a plateau at >3.0 mg/kg. The best response was stable disease in 6/27 (22%) patients.SL-172154 was tolerable as monotherapy and induced, dose-dependent, and cyclical immune cell activation, increases in multiple serum cytokines, and trafficking of CD40-positive B cells and monocytes following each infusion. The safety, PK, and PD activity support 3.0 mg/kg as a safe and pharmacologically active dose.NCT04406623.© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Moxibustion inhibits the macrophage M1 polarization toll-like receptor 4/myeloid differentiation factor 88/nuclear factor kappa B signaling pathway by regulating T-cell immunoglobulin and mucin-containing protein-3 in rheumatoid arthritisKun, Yumei, Yanding
et alJ Tradit Chin Med (2024) 44 (6), 1227-1235
Abstract: To explore whether moxibustion exerts therapeutic effects on rheumatoid arthritis (RA) by regulating the expression of T-cell immunoglobulin and mucin-containing protein-3 (TIM-3) and subsequently modulating the macrophage M1 polarization toll-like receptor 4 (TLR4)-myeloid differentiation factor 88 (MyD88)-nuclear factor kappa B (NF-κB) signaling pathway.We utilized moxibustion treatment in RA rat models using the Zusanli (ST36) and Shenshu (BL23) acupoints. Hematoxylin and eosin (HE) staining was used to observe the pathological changes of the synovial tissue under a section light microscope, and pathological scoring was performed according to the grading standard of the degree of synovial tissue disease. Enzyme-linked immunosorbent assay (ELISA) was applied to verify the efficacy of moxibustion in reducing inflammation. Quantitative real-time polymerase chain reaction (qRT-PCR) was used to detect the expression of the TIM-3/TLR4-MyD88-NF-κB signaling pathway-related molecules, and Western blot was used to detect the contents of synovial NF-κB.We established the Freund's complete adjuvant (FCA)-induced RA model in rats. The expression level of M1 polarization signaling pathway TLR4-MyD88-NF-κB and the inflammatory factors interleukin-12(IL-12), tumor necrosis factor alpha (TNF-α), and tumor necrosis factor beta (TNF-β) were significantly increased in the RA model. After moxibustion treatment, the expression level of TLR4-MyD88-NF-κB was significantly decreased, and the inflammatory factors IL-12, TNF-α, and TNF-β were decreased, but the expression level was significantly increased in the RA model. When TIM-3 expression was inhibited, the expression level of TLR4-MyD88-NF-κB, and the inflammatory factors IL-12, TNF-α, and TNF-β were not suppressed, even after moxibustion treatment.Moxibustion regulates the key target TIM-3 by acting on the Zusanli (ST36) and Shenshu (BL23) points, thereby inhibiting the M1 polarization of macrophages; that is, it inhibits the TLR4-MyD88-NF-κB signaling pathway, and finally achieves alleviation of pathological changes and anti-inflammatory effects.
Elucidating the role of T-Reg related cytokines: serum transforming growth factor beta and interleukin-35 in alopecia areataYuksek, Gonul, Kartal
et alArch Dermatol Res (2024) 316 (6), 205
Abstract: Previous studies demonstrated that Th1 cytokines like IL-2, IL-12 and IFN-γ have initiatory role in alopecia areata (AA) and positive correlation with disease severity. They informed that serum levels of Th17 cytokines, IL-17, IL-22, IL-23 increased in active AA patients and corelated, particularly IL-17, with disease severity. In recent reports it was showed the balance between Th17 and Treg cells is crucial for maintaining tolerance to self-antigens, and an imbalance towards Th17 may contribute to the development of autoimmune diseases like AA. But research on serum Treg markers in AA is limited. It was aimed to investigate whether the Treg cells have a role in the pathogenesis of AA analyzing the serum levels of Treg cytokines IL-35 and TGF-β in the patients with AA. 42 AA patients and 38 healthy controls were enrolled. Patient demographics, clinical data, disease severity assessed by Severity of Alopecia Tool (SALT) scores were recorded. Serum samples were collected and analyzed for TGF-β and IL-35 levels using ELISA kits. The cytokine levels in both groups were statistically compared. Their relation with parameters of demographic and severity of disease was evaluated. The patient and control groups had no statistically significant difference, there was 71.4% males and 28.6% females in patient group, while the control group had 63.2% males and 36.8% females, Severity analysis classified 18 patients with mild AA, 19 with moderate AA, and 5 with alopecia totalis/areata universalis. While TGF-β levels exhibited no significant difference between groups, IL-35 levels were significantly elevated in AA patients (p = 0.002). Logistic regression identified IL-35 as a significant parameter influencing disease status (OR = 1.055). Correlation analysis revealed a weak positive correlation between patient age and IL-35 levels (r = 0.436; p = 0.004). Notably, IL-35 levels displayed a significant decrease in individuals with antinuclear antibody (ANA) positivity. No correlations were identified between cytokine levels and disease severity, prognosis, or disease activity. Elevated IL-35 levels suggest that IL-35 and specific Treg cell subsets can play a role in AA pathogenesis. The nuanced roles of TGF-β and IL-35 highlight the need for comprehensive studies to interpret their implications in the complex immunopathogenesis of AA. These findings open avenues for further research, positioning IL-35 as a prospective target for investigating and potentially intervening in AA pathogenesis.© 2024. The Author(s), under exclusive licence to Springer-Verlag GmbH Germany, part of Springer Nature.
Potential mitigation of titanium dioxide nanoparticles against 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced colitis through inhibiting the canonical NF-κB pathwayGao, He, Duan
et alNanoImpact (2024) 34, 100512
Abstract: Titanium dioxide nanoparticles (TiO2 NPs) have been widely employed in various industry fields, which makes consumers concerned about their health impact. Our previous work displayed that TiO2 NPs participated in the mitigation of TNBS-induced colitis, but the mechanism is still unknown. This work aimed to explore the role of oxidative stress and NF-κB pathway in the effect of TiO2 NPs on TNBS-induced colitis. The results showed that TiO2 NPs administration reduced the DAI score of colitis mice after TNBS enema. TiO2 NPs did not alter oxidative stress status (GSH/GSSG), but repaired the gut dysbacteriosis and inhibited the canonical NF-κB pathway activation in TNBS-induced colitis mice, manifested as a decrease in pathogenic bacteria and an increase in beneficial bacteria, as well as down-regulation of toll-like receptors (TLRs), IKKα, IKKβ, p65 and pro-inflammatory cytokines (IL-1β, IL-6, TNF-α and IFN-γ) in mRNA level, and the increased transcription of anti-inflammatory cytokines (IL-10, TGF-β, and IL-12), along with the declined protein level of TNF-α in TiO2 NPs treated colitis mice. The present study suggested that oral TiO2 NPs administration inhibited the canonical NF-κB pathway activation by repairing gut dysbacteriosis, which made a predominant role in alleviating colitis. These findings provided a new perspective for exploring the safety of TiO2 NPs.Copyright © 2024 Elsevier B.V. All rights reserved.

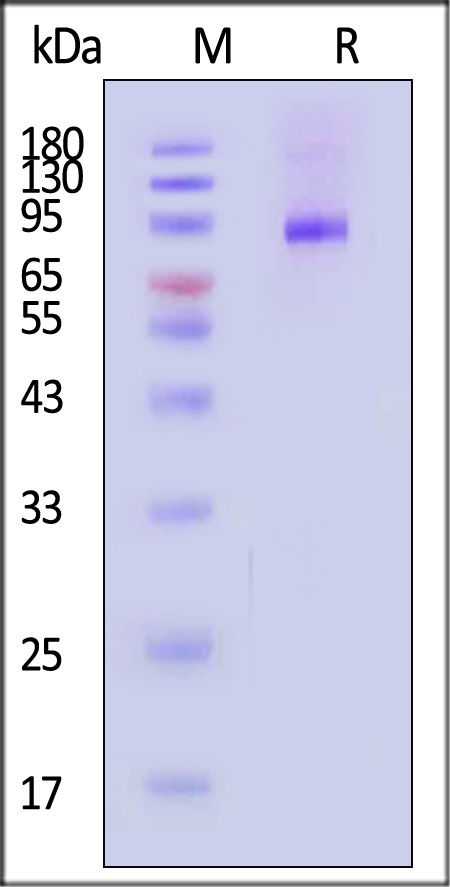

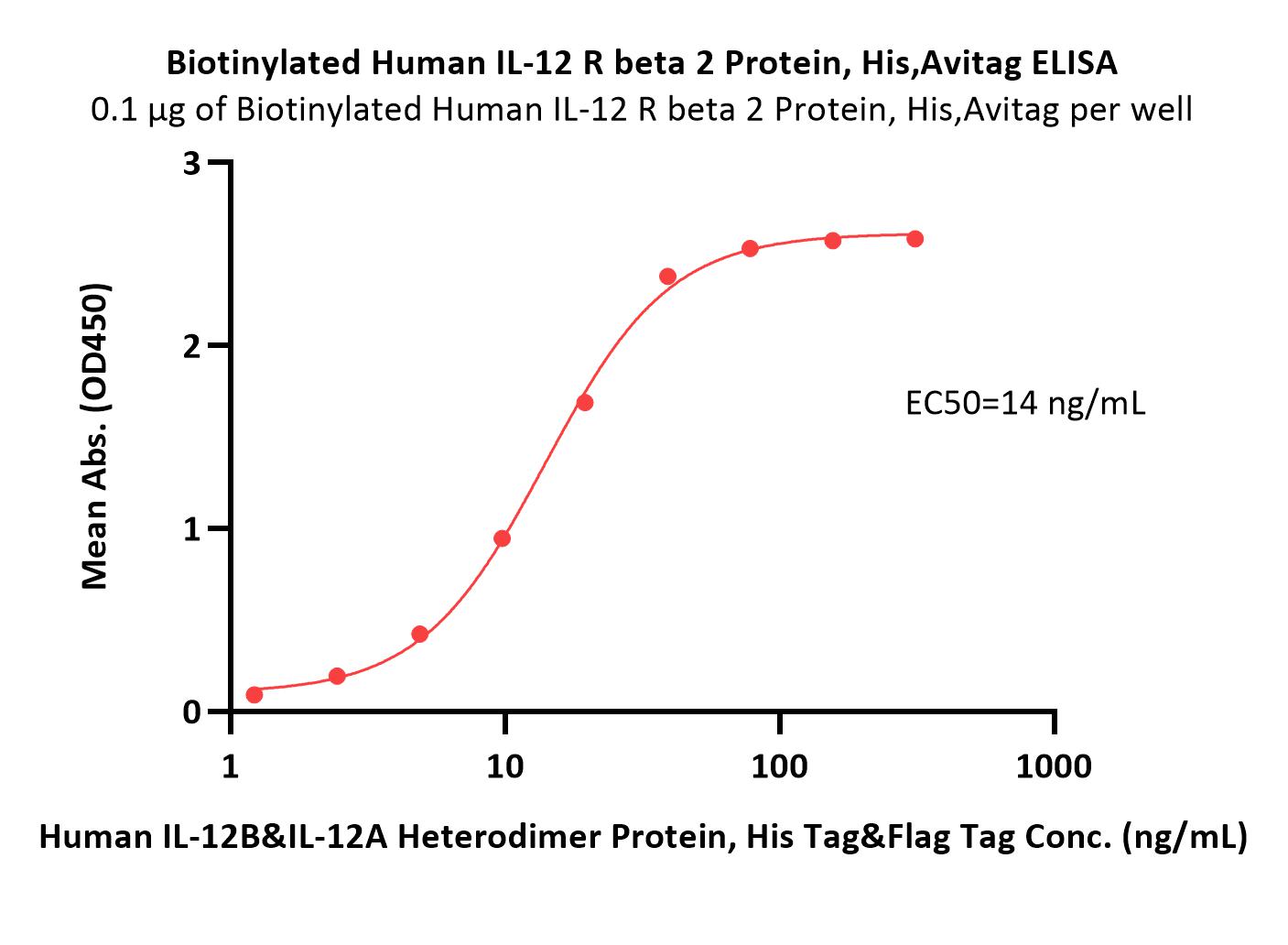























































 膜杰作
膜杰作 Star Staining
Star Staining















