Contribution of cuproptosis and immune-related genes to idiopathic pulmonary fibrosis diseaseJin, Li, Li
et alFront Immunol (2025) 16, 1458341
Abstract: Idiopathic pulmonary fibrosis (IPF) is a degenerative respiratory condition characterized by significant mortality rates and a scarcity of available treatment alternatives. Cuproptosis, a novel form of copper-induced cell death, has garnered attention for its potential implications. The study aimed to explore the diagnostic value of cuproptosis-related hub genes in patients with IPF. Additionally, multiple bioinformatics analyses were employed to identify immune-related biomarkers associated with the diagnosis of IPF, offering valuable insights for future treatment strategies.Four microarray datasets were selected from the Gene Expression Omnibus (GEO) collection for screening. Differentially expressed genes (DEGs) associated with IPF were analyzed. Additionally, weighted gene coexpression network analysis (WGCNA) was employed to identify the DEGs most associated with IPF. Ultimately, we analyzed five cuproptosis-related hub genes and assessed their diagnostic value for IPF in both the training and validation sets. Additionally, four immune-related hub genes were screened using a protein-protein interaction (PPI) network and evaluated through the receiver operating characteristic (ROC) curve. Lastly, single-cell RNA-seq was employed to further investigate differential gene distribution.We identified a total of 92 DEGs. Bioinformatics analysis highlighted five cuproptosis-related genes as candidate biomarkers, including three upregulated genes (CFH, STEAP1, and HDC) and two downregulated genes (NUDT16 and FMO5). The diagnostic accuracy of these five genes in the cohort was confirmed to be reliable. Additionally, we identified four immune-related hub genes that demonstrated strong diagnostic performance for IPF, with CXCL12 showing an AUROC of 0.90. We also examined the relationship between these four genes and immune cells. CXCL12 was significantly negatively associated with neutrophils, while CXCR2 was associated exclusively with neutrophils, consistent with our single-cell sequencing results. CTSG showed a primarily positive association with follicular helper T, and SPP1 was most strongly associated with macrophages. Finally, our single-cell sequencing data revealed that in patients with IPF, CXCL12 was highly expressed in the endothelial cell subset (ECs), while SPP1 exhibited high expression in multiple cellular populations. The expression of the CTSG showed statistically significant differences in monocyte macrophages.The research methodically depicted the intricate interplay among five cuproptosis-related genes, four immune-related hub genes, and IPF, offering new ideas for diagnosing and treating patients with IPF.Copyright © 2025 Jin, Li, Li, Zhang, Zheng, Feng, Li, Zheng, Nie, Liang and Wang.
Prostate Extracellular Vesicles and Prognostic Biomarkers of Clinically Significant Prostate Cancer: A Prospective Single-Institution Pilot StudyBritton, Andrews, Arafa
et alProstate (2025) 85 (6), 594-602
Abstract: Commercial biomarkers and multiparametric MRI (mpMRI) have been utilized to triage men with elevated prostate specific antigen (PSA) and determine patients most likely to harbor clinically-significant prostate cancer (csPCa). We studied combinations of mpMRI, PSA-based and novel extracellular vesicle (EV)-based biomarkers to determine the optimal pre-biopsy testing to predict csPCa at biopsy.Men presenting with elevated PSA (≥ 2 ng/mL) were prospectively enrolled and all men underwent clinically indicated mpMRI and blinded study blood draws to determine PSA, prostate health index (PHI) scoring, and EV serum levels. MRI-fusion transperineal prostate biopsy was performed in all patients. Sensitivity, specificity, positive predictive value, and negative predictive value were calculated, and receiver operator characteristic (ROC) curves were constructed. Bootstrapping analysis was performed to provide more accurate assessment of predictive abilities.Ultimately, 175 consecutive men were prospectively enrolled. Median age in the study population was 65 years. Combinations of biomarkers and MRI demonstrated better predictive ability for csPCa on biopsy than individual modalities. Predictive ability was greatest for PHI density (PHID)-mpMRI with an AUC of 0.86 (95% CI: 0.80-0.92) while combinations of PSA density -mpMRI (AUC: 0.81; 95% CI: 0.73-0.89) and STEAP1-mpMRI (AUC: 0.77; 95% CI: 0.70-0.86) demonstrated similar ability to predict csPCa. The study is limited by small, predominantly white patient cohort and requires external validation.Inclusion of prostate density with biomarkers increases prognostic ability for detecting csPCa. EV density can refine prediction of csPCa and in combination with PHID-mpMRI leads to superior specificity, thereby decreasing unnecessary biopsies.© 2025 Wiley Periodicals LLC.
A novel prostate cancer-specific fluorescent probe based on extracellular vesicles targeting STEAP1 applied in fluorescence guided surgerySun, Xia, Xu
et alJ Control Release (2025) 380, 199-218
Abstract: Radical prostatectomy with pelvic lymph node dissection is the best treatment for intermediate- to high-risk localized prostate cancer (PCa). However, conventional white light surgery has difficulties in identifying tumor boundary and micrometastases intraoperatively. Fluorescence guided surgery (FGS) can solve the above difficulties, but lacks tumor-specific near-infrared fluorescent (NIRF) probes in PCa. STEAP1 was an ideal target in PCa treatment and imaging. Here, we constructed a PCa specific fluorescent probe based on extracellular vesicles targeting STEAP1 (AS-EVs) loaded with NIRF dye S0456 and evaluated its preclinical profiles. In vitro and in vivo studies both showed S0456@AS-EVs was safe and showed strong targeting ability to PCa in various mice xenograft models. S0456@AS-EVs could clear rapidly from blood (half-time of 4.29 h) and retain in the STEAP1 positive tumor tissues for more than 72 h with the highest tumor background ratio (TBR) of 3:1, which was superior to ICG, free S0456, ICG@Ctrl-EVs and S0456@Ctrl-EVs (p < 0.01). Finally, S0456@AS-EVs was applied in FGS on intramuscular model, and the tumors were resected under white light and fluorescence respectively. Compared with white light surgery, mice undergoing FGS had lower positive margin rate and better postoperative survival (p = 0.0342).Copyright © 2025 The Authors. Published by Elsevier B.V. All rights reserved.
Advances in CAR T cell therapy: antigen selection, modifications, and current trials for solid tumorsKhan, Choi, Veena
et alFront Immunol (2024) 15, 1489827
Abstract: Chimeric antigen receptor (CAR) T cell therapy has revolutionized the treatment of hematologic malignancies, achieving remarkable clinical success with FDA-approved therapies targeting CD19 and BCMA. However, the extension of these successes to solid tumors remains limited due to several intrinsic challenges, including antigen heterogeneity and immunosuppressive tumor microenvironments. In this review, we provide a comprehensive overview of recent advances in CAR T cell therapy aimed at overcoming these obstacles. We discuss the importance of antigen identification by emphasizing the identification of tumor-specific and tumor-associated antigens and the development of CAR T therapies targeting these antigens. Furthermore, we highlight key structural innovations, including cytokine-armored CARs, protease-regulated CARs, and CARs engineered with chemokine receptors, to enhance tumor infiltration and activity within the immunosuppressive microenvironment. Additionally, novel manufacturing approaches, such as the Sleeping Beauty transposon system, mRNA-based CAR transfection, and in vivo CAR T cell production, are discussed as scalable solution to improve the accessibility of CAR T cell therapies. Finally, we address critical therapeutic limitations, including cytokine release syndrome (CRS), immune effector cell-associated neurotoxicity syndrome (ICANS), and suboptimal persistence of CAR T cells. An examination of emerging strategies for countering these limitations reveals that CRISPR-Cas9-mediated genetic modifications and combination therapies utilizing checkpoint inhibitors can improve CAR T cell functionality and durability. By integrating insights from preclinical models, clinical trials, and innovative engineering approaches, this review addresses advances in CAR T cell therapies and their performance in solid tumors.Copyright © 2025 Khan, Choi, Veena, Lee and Shin.


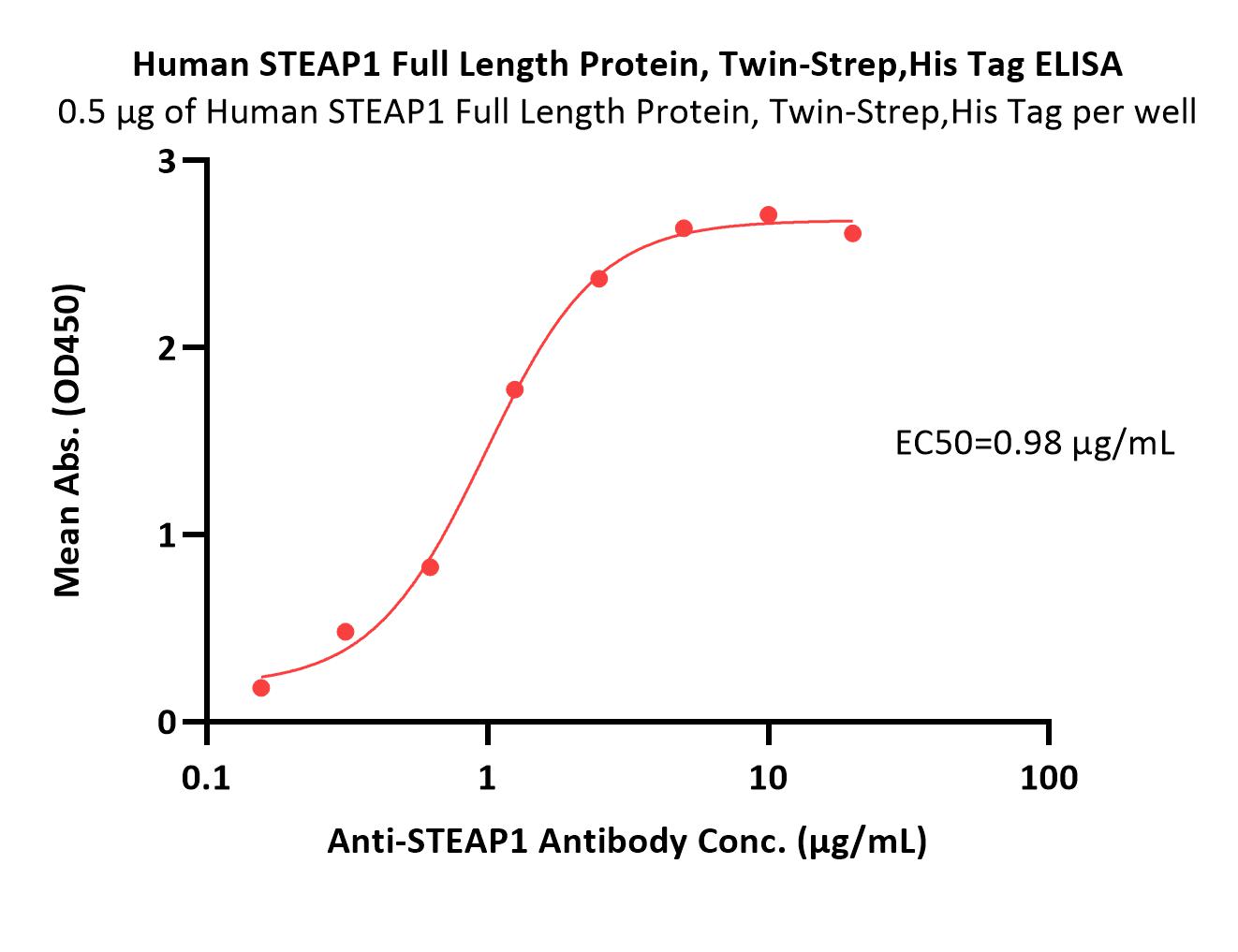

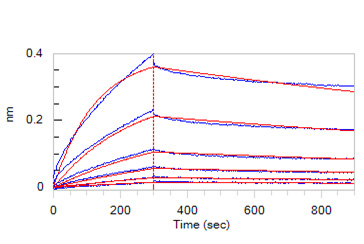























































 膜杰作
膜杰作 Star Staining
Star Staining















