| Product | Size | Amount |
| GMP ActiveMax® Human T cell Activation/Expansion CD3/CD28 Beads | 10 mg | 1 × 10⁸ beads |
| 100 mg (10 mg × 10) | 1 × 10⁹ beads | |
| 400 mg (10 mg × 40) | 4 × 10⁹ beads |
优势特色(Features)
- Designed in ISO 9001:2015 and ISO 13485:2016 certified facility
- Manufactured and QC tested under a GMP compliance factory
- FDA DMF filed
- Animal-Free materials
- Beta-lactam materials free
- Batch-to-batch consistency
- GMP grade antibodies as raw materials with strict virus removal steps and testing
- GMP grade recombinant HSA as excipient
- Stringent quality control tests
背景(Background)
GMP ActiveMax® Human T cell Activation/Expansion CD3/CD28 Beads are uniform 5.5 μm of magnetic beads coated with an optimized mixture of GMP grade mouse monoclonal antibodies against the CD3 and CD28, mimicking in vivo stimulation by APCs. GMP ActiveMax® Human T cell Activation/Expansion CD3/CD28 Beads are Manufactured under a GMP compliance factory with animal free raw materials, and tested under GMP guidelines. GMP ActiveMax® Human T cell Activation/Expansion CD3/CD28 Beads are intended for the in vitro isolation, stimulation and expansion of purified T cell populations of, for example, CD3+T cells, CD4+ T cells, CD8+ T cells or human PBMC for cell based clinical and preclinical research.
存储(Storage)
The product MUST be stored at 2-8°C upon receipt; This product is stable after storage at 2-8°C for 5 years under sterile conditions.
无菌(Sterility)
The sterility testing was performed by membrane filtration method described in CP<1101>, USP<71> and Eur. Ph. 2.6.1.
内毒素(Endotoxin)
Less than 0.5 EU per mL by the LAL method.
制剂(Formulation)
GMP ActiveMax Human T cell Activation/Expansion CD3/CD28 Beads is supplied with 5x10⁷ beads/mL in PBS, pH 7.4, with 0.1% recombinant human serum albumin (recombinant HSA).
典型数据-Typical Data Please refer to DS document for the assay protocol.
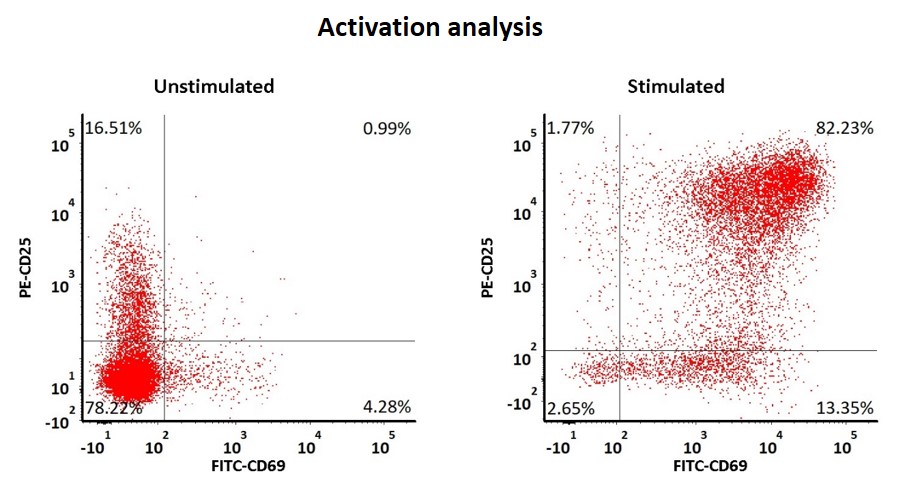
The human T cells were stimulated with GMP ActiveMax® Human T cell Activation/Expansion CD3/CD28 Beads (Cat. No. GMP-MBS001) for 24hrs, and the activation was assessed by measuring expression of both activation markers CD25 and CD69 expression on the T cells surface by stanning with PE labeled anti-human CD25 antibody and FITC labeled anti-human CD69 antibody respectively (QC tested).
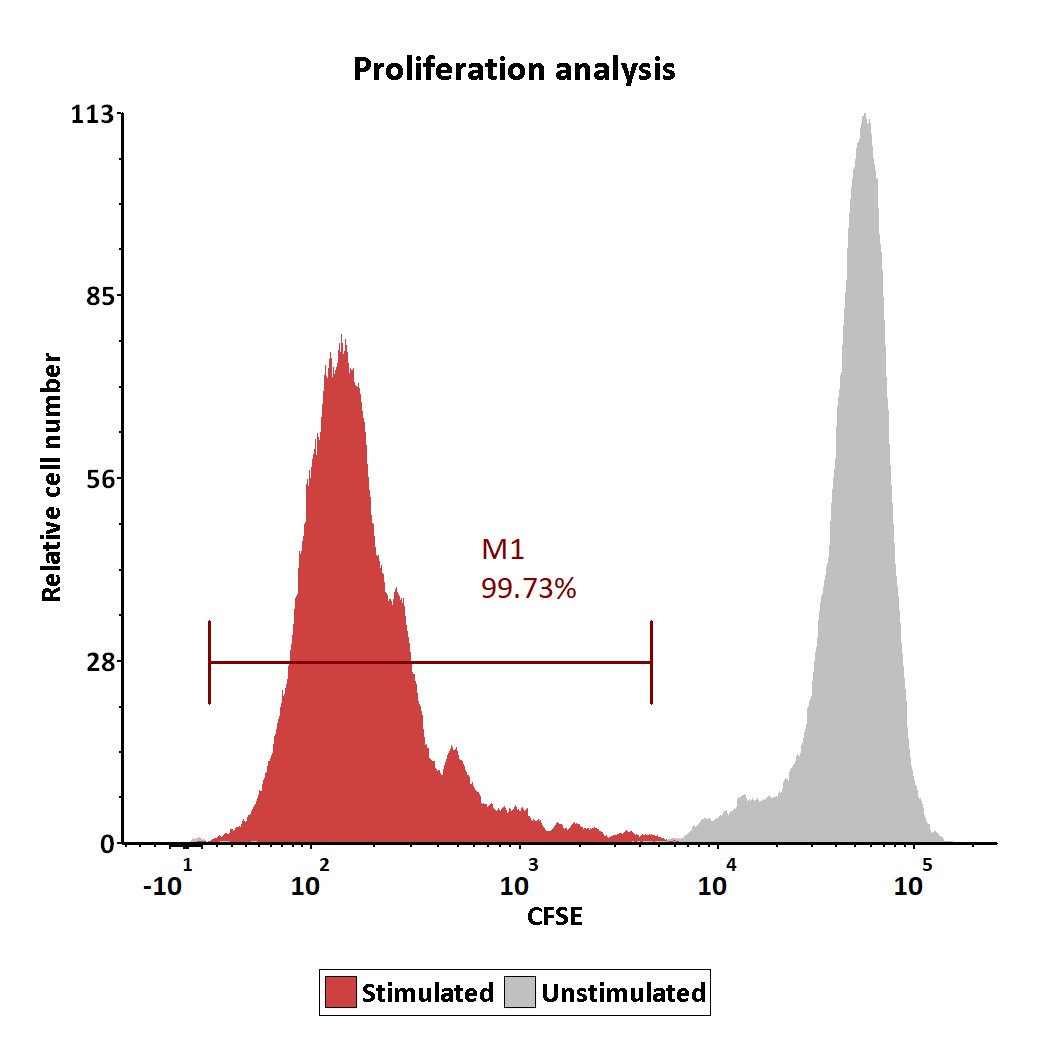
The human T cells were labeled with carboxy fluorescein succinimidyl ester (CFSE) and stimulated with GMP ActiveMax® Human T cell Activation/Expansion CD3/CD28 Beads (Cat. No. GMP-MBS001), and then the proliferation of the T cells was assessed with CFSE dilution assay by flow cytometry on day 5 after stimulation (QC tested).
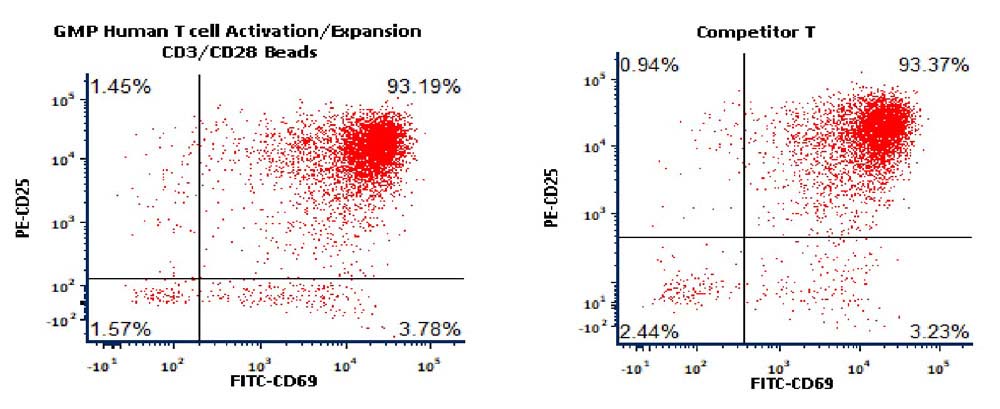
Activation of the purified human T Cells. The purified human T cells were activated using Human T cell Activation/Expansion CD3/CD28 Beads, (ACRO, Cat. No. GMP-MBS001) and Competitor-Beads respectively for 24 hours with CTS Optimizer Medium. Cells were fluorescently stained using PE labeled anti-human CD25 antibody and labeled FITC anti-human CD69 antibody and analyzed by flow cytometry.
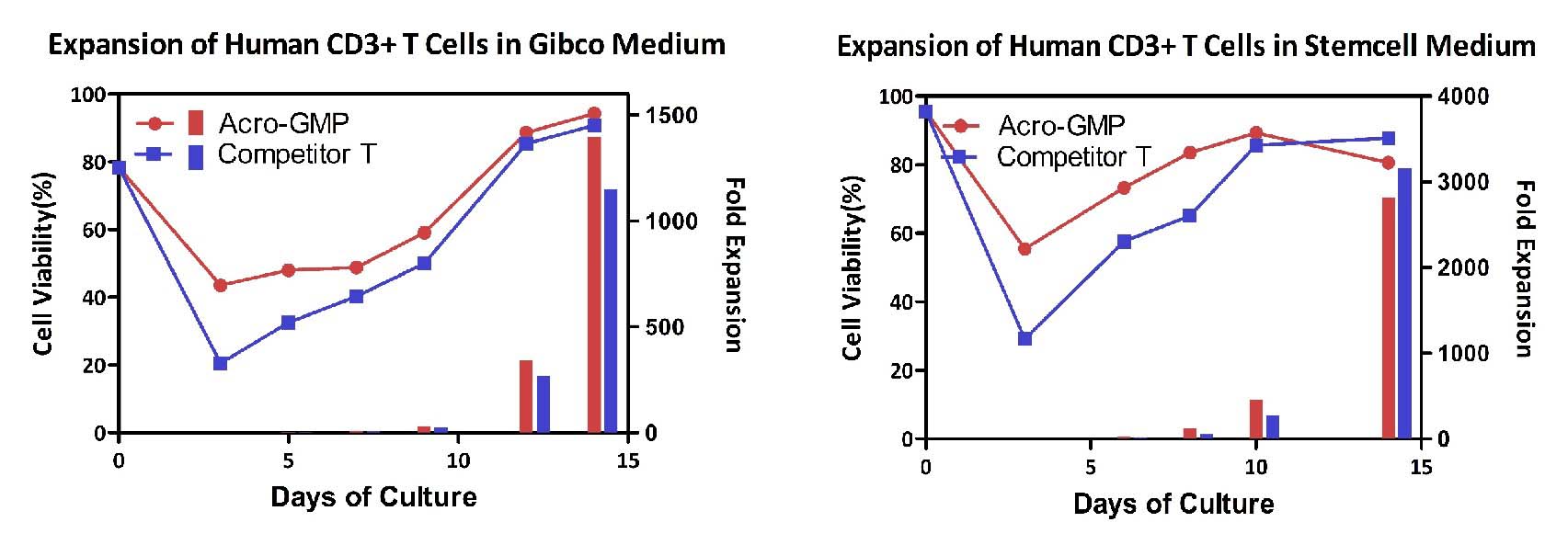
Expansion of the human CD3+T cells. Human T cells using ACROBiosystems CD3/CD28 Beads (ACRO, Cat. No. GMP-MBS001) were expanded under two different medium, respectively. Expansion was performed for two weeks, showing that ACROBiosystems’ beads showing better proliferative abilities and comparable competitive ideas compared with competitor product.
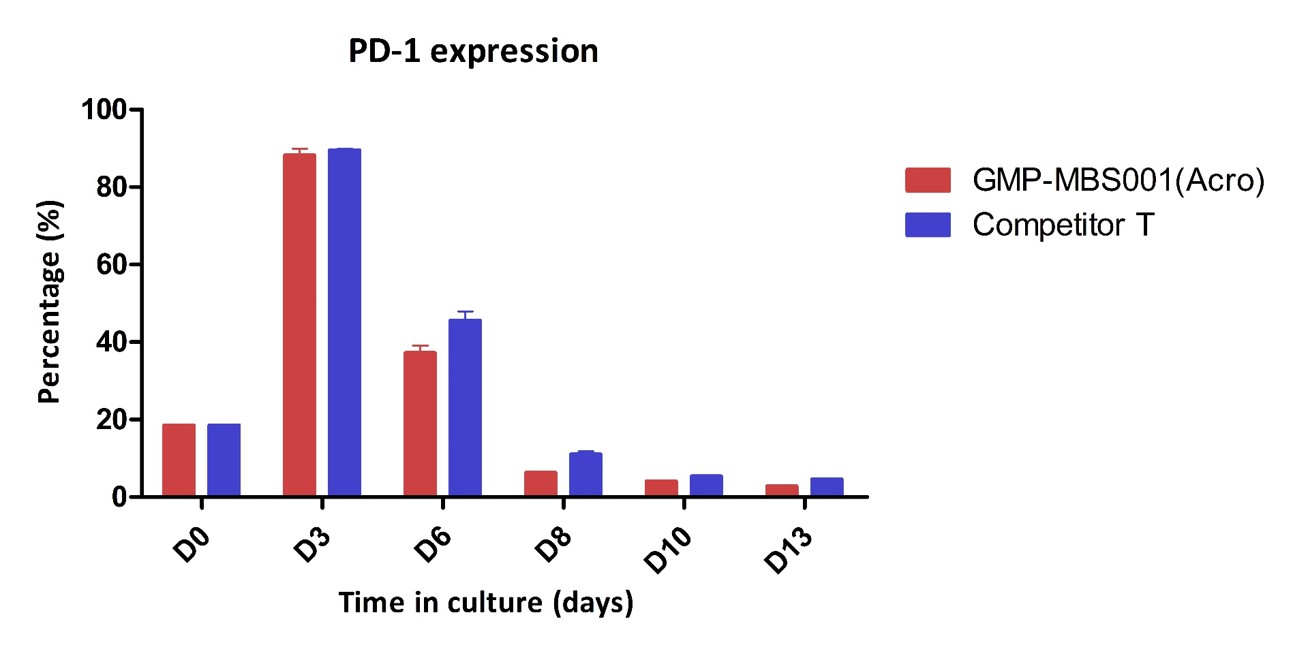
PD-1 expression of the activated human T Cells. The purified human T cells were stimulated using Human T cell Activation/Expansion CD3/CD28 Beads at a ratio of 1:1 beads-to-cells. Cells were expanded in T cell culture medium supplemented with 4ng/mL of rhIL-2 Protein. Activated T cells were expanded for up to 8 days with low PD-1 expression.
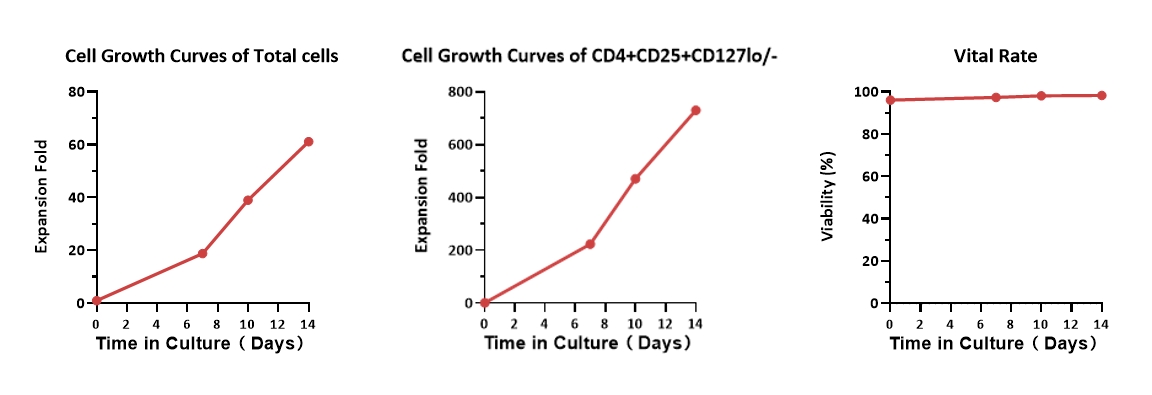
Human CD4+ cells were activated with GMP ActiveMax Human T cell Activation/Expansion CD3/CD28 Beads (Cat. No. GMP-MBS001), and cultured with GMP Human IL-2 Protein (Cat. No. GMP-L02H14), GMP Human TGF-Beta 1 Protein (Cat. No. GMP-TG1H25), Rapamycin, all-trans retinoic acid and sodium butyrate in CelThrea™ GMP T Cell Expansion Medium (Cat. No. GMP-CM3101) for two weeks. The result shows that CelThrea™ GMP T Cell Expansion Medium with GMP ActiveMax Human T cell Activation/Expansion CD3/CD28 Beads, GMP Human IL-2 Protein and GMP Human TGF-Beta 1 Protein can promote the expansion of Treg cells with a reasonable cell viability.
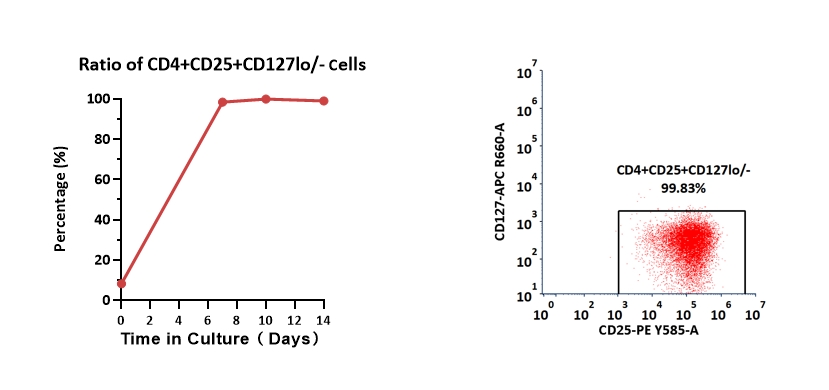
Human CD4+ cells were activated with GMP ActiveMax Human T cell Activation/Expansion CD3/CD28 Beads (Cat. No. GMP-MBS001), and cultured with GMP Human IL-2 Protein (Cat. No. GMP-L02H14), GMP Human TGF-Beta 1 Protein (Cat. No. GMP-TG1H25), Rapamycin, all-trans retinoic acid and sodium butyrate in CelThrea™ GMP T Cell Expansion Medium (Cat. No. GMP-CM3101) for two weeks. The result shows that CelThrea™ GMP T Cell Expansion Medium with GMP ActiveMax® Human T cell Activation/Expansion CD3/CD28 Beads, GMP Human IL-2 Protein and GMP Human TGF-Beta 1 Protein can increase the percentage of the CD4+CD25+CD127lo/-. With the post-culture time, the proportion of CD4+CD25+CD127lo/- cells remained unchanged.
稳定性(Stability)
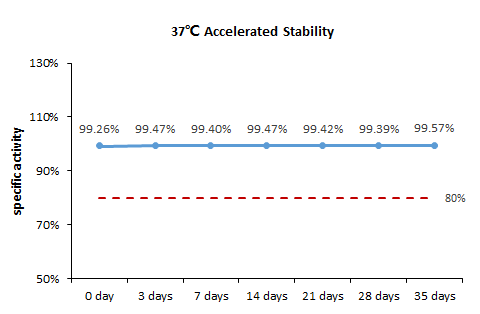
The Cell based assay shows that GMP ActiveMax® Human T cell Expansion CD3/CD28 Beads(GMP-MBS001) is stable at 37℃ for 35 days.
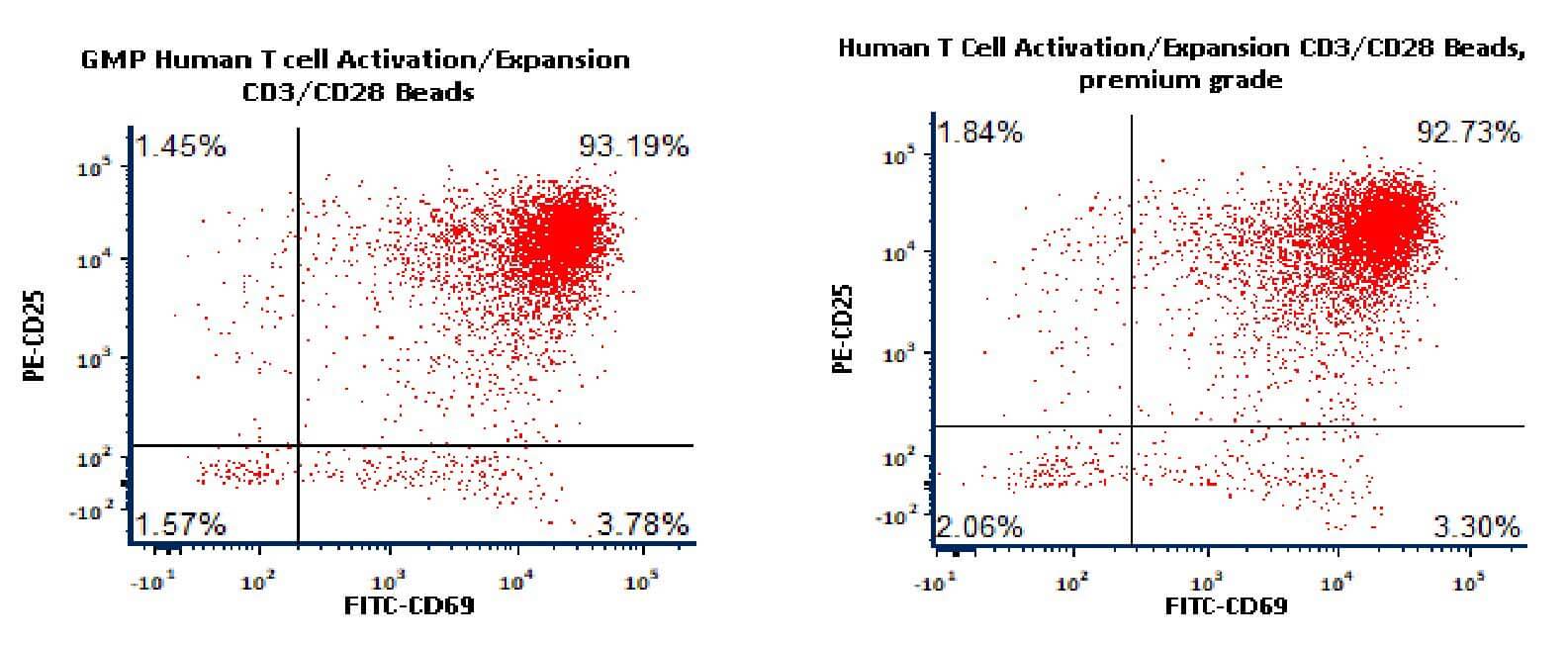
Activation of the purified human T Cells. The purified human T cells were activated using Human T cell Activation/Expansion CD3/CD28 Beads, (ACRO, Cat. No. GMP-MBS001/MBS-C001) respectively for 24 hours with CTS Optimizer Medium. Cells were fluorescently stained using PE labeled anti-human CD25 antibody and labeled FITC anti-human CD69 antibody and analyzed by flow cytometry.
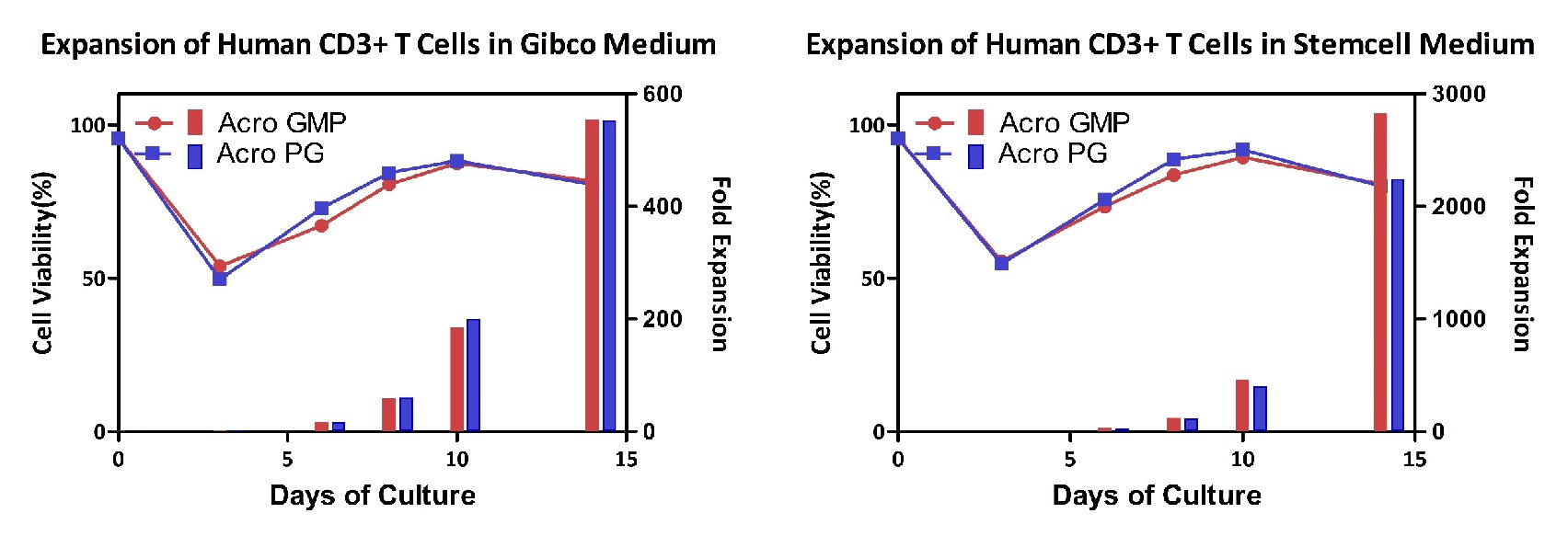
Expansion of the human CD3+T cells. Human T cells using ACROBiosystems CD3/CD28 Beads (ACRO, Cat. No. GMP-MBS001/MBS-C001) were expanded under two different medium, respectively. Expansion was performed for two weeks, showing that ACROBiosystems’ GMP and PG beads showing similar proliferative abilities.
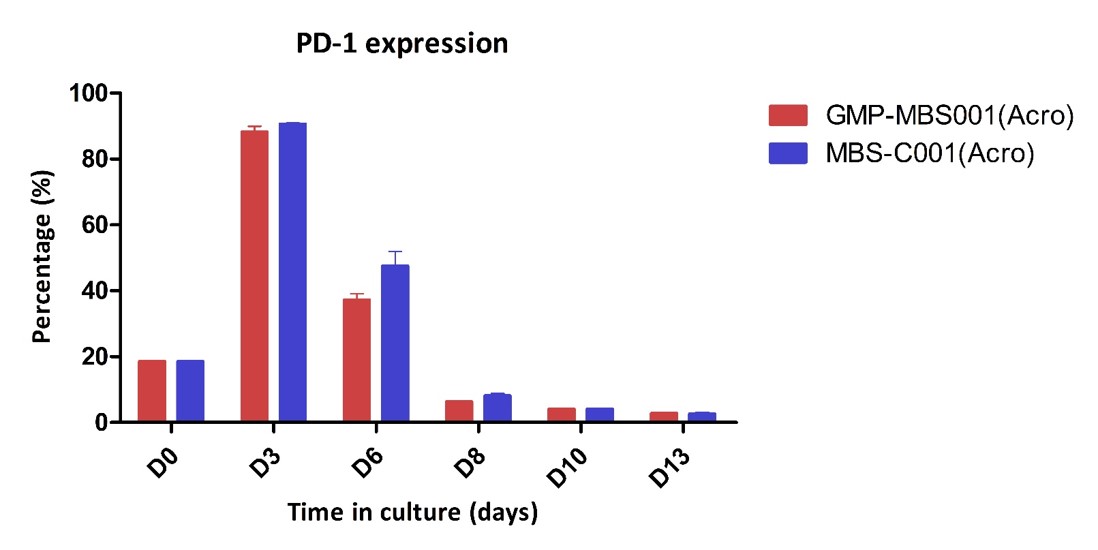
PD-1 expression of the activated human T Cells. The purified human T cells were stimulated using Human T cell Activation/Expansion CD3/CD28 Beads at a ratio of 1:1 beads-to-cells. Cells were expanded in T cell culture medium supplemented with 4ng/mL of rhIL-2 Protein. Activated T cells were expanded for up to 8 days with low PD-1 expression.
MANUFACTURING SPECIFICATIONS
ACROBiosystems GMP grade products are produced under a quality management system and in compliance with relevant guidelines: Ph. Eur General Chapter 5.2.12 Raw materials of biological origin for the production of cell-based and gene therapy medicinal products; USP <92> Growth Factors and Cytokines Used in Cell Therapy Manufacturing; USP <1043> Ancillary Materials for Cell, Gene, and Tissue-Engineered Products; ISO/TS 20399-1:2018, Biotechnology - Ancillary Materials Present During the Production of Cellular Therapeutic Products.
ACROBiosystems Quality Management System Contents:
- Designed in ISO 9001:2015 and ISO 13485:2016 certified facility, Manufactured and QC tested under a GMP compliance factory.
- Animal-Free materials
- Materials purchased from the approved suppliers by QA
- Qualified personnel
- Quality-related documents review and approve by QA
- Fully batch production and control records
- Equipment maintenance and calibration
- Validation of analytical procedures
- Stability studies conducted
- Comprehensive regulatory support files
Request For Regulatory Support Files(RSF) Request For DMF
ACROBiosystems provide rigorous quality control tests (fully validated equipment, processes and test methods) on our GMP grade products to ensure that they meet stringent standards in terms of purity, safety, activity and inter-batch stability, and each bulk QC lot mainly contains the following specific information:
- SDS-PAGE
- Protein content
- Endotoxin level
- Residual Host Cell DNA content
- Residual Host Cell Protein content
- Biological activity analysis
- Microbial testing
- Mycoplasma testing
- In vitro virus assay
- Batch-to-batch consistency
DISCLAIMER
ACROBiosystems GMP grade products are designed for research, manufacturing use or ex vivo use. CAUTION: Not intended for direct human use.
TERMS AND CONDITIONS
All products are warranted to meet ACROBiosystems Inc.’s (“ACRO”) published specifications when used under normal laboratory conditions.
ACRO DOES NOT MAKE ANY OTHER WARRANTY OR REPRESENTATION WHATSOEVER, WHETHER EXPRESS OR IMPLIED, WITH RESPECT TO ITS PRODUCTS. IN PARTICULAR, ACRO DOES NOT MAKE ANY WARRANTY OF SUITABILITY, NONINFRINGEMENT, MERCHANTABILITY OR FITNESS FOR ANY PARTICULAR PURPOSE.
NOT WITH STANDING ANY OTHER PROVISIONS OF THESE TERMS AND/OR ANY OTHER AGREEMENT BETWEEN ACRO AND PURCHASER FOR THE PURCAHSE OF THE PRODUCTS, ACRO’S TOTAL LIABILITY TO PURCHASER ARISING FROM OR IN RELATION TO THESE TERMS, AN AGREEMENT BETWEEN THE PARTIES OR THE PRODUCTS, WHETHER ARISING IN CONTRACT, TORT OR OTHERWISE SHALL BE LIMITED TO THE TOTAL AMOUNT PAID BY PURCHASER TO ACRO FOR THE RELEVANT PRODUCTS. IN NO EVENT WILL ACRO BE LIABLE FOR THE COST OF PROCUREMENT OF SUBSTITUTE GOODS.
END USER TERMS OF USE OF PRODUCT
The following terms are offered to you upon your acceptance of these End User Terms of Use of Product. By using this product, you indicate your acknowledgment and agreement to these End User Terms of Use of Product. If you do not agree to be bound by and comply with all of the provisions of these End User Terms of Use of Product, you should contact your supplier of the product and make arrangements to return the product.
The End User is aware that ACROBiosystems Inc. and its affiliate (“ACRO”) sell GMP grade products designed for research, manufacturing use or ex vivo use and not intended for human in vivo applications. The End User further agrees, as a condition of the sales of ACRO’s GMP grade products that: a) the End User will not use this GMP grade product in any procedure wherein the product may be directly or indirectly administered to humans, unless the End User has obtained, or prior to their use will have obtained, an Investigational New Drug (IND) exemption from the FDA and will use the product only in accordance with the protocols of such IND and of the Institutional Review Board overseeing the proposed research, or b) the End User will use the products outside of the United States in accordance with the protocols of research approved by the applicable review board or authorized ethics committee and regulatory agencies to which the End User is subject to in their territory.






















































 膜杰作
膜杰作 Star Staining
Star Staining






 & Cat.No.
& Cat.No. 




