产品概述(Product Details)
ClinMax™ Human IL-17A / CTLA8 ELISA Kit is a ready-to-use immunoassay kit, specifically designed to quantitate natural and recombinant human IL-17A / CTLA8 that is present in biological samples, such as human serum, plasma, and cell culture supernatants. Our ClinMax™ ELISA Kit provides several benefits:
- Standards to calibrate with NIBSC/WHO standards for comparable results.
- Fully validation in biologic samples for detection range, sensitivity, inter- and intra-plate CV, recovery, dilution linearity, specificity, and matrix effects to ensure reliable results according to ICH M10 guideline.
- High-quality antibody pairs and protein standards, along with rigorous quality control, to guarantee consistent results across different batches.
- Simplified and straightforward protocols and ready-to-use reagents to save assay time.
应用说明(Application)
The kit is developed for quantitative detection of natural and recombinant human IL-17A / CTLA8 in serum, plasma and cell culture supernatants.
It is for research use only.
流程图(Workflow)

关键信息(Key Features)
| Analyte | IL-17A/CTLA8 |
| Assay Type | Sandwich-ELISA |
| Reactivity | Human |
| Sensitivity | <6pg/mL |
| Range | 31.25 pg/mL-2000 pg/ml |
| Assay Time | 2hr |
| Sample Type | Cell Culture Supernatants, Plasma, Serum. |
| Sample volume | 50 μL |
| Format | 96-wells plate breakable into 12 x 8 wells strips |
Elevate your research experience with our Cytokine/Biomarker Detection Kits, where accuracy, reliability, and ease of use are converging to deliver exceptional results.
存储(Storage)
Keep the unopened kit stored at 2-8 °C. Avoid using the kit beyond its expiration date. The opened kit should be stored per components table. The shelf life is 30 days from the date of opening.
典型数据-Typical Data Please refer to DS document for the assay protocol.

For each experiment, a standard curve needs to be set for each microplate, and the specific OD value may vary depending on different laboratories, testers, or equipment. The following example data is for reference only. The sample concentration was calculated based on the results of the standard curve. The minimum detectable concentration of IL-17A is less than 6 pg/mL.
验证(Validation)
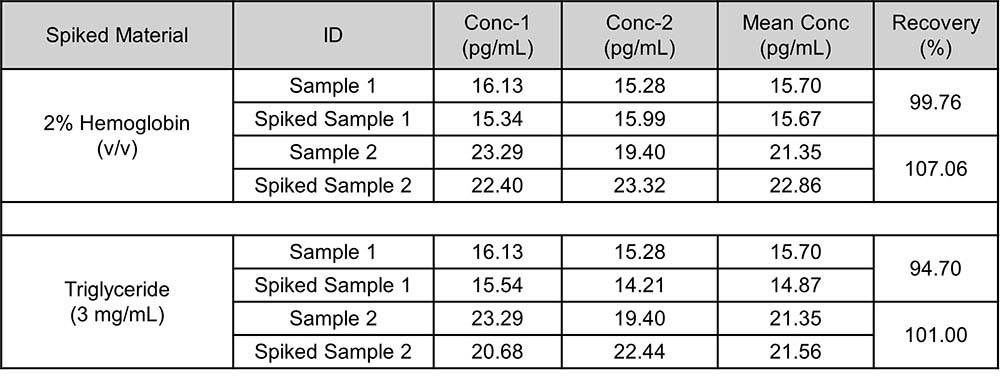
基质效应(Matrix Effect)
3 types of materials were tested to observe if there were matrix effect (interference). If the concentration of hemoglobin (simulated hemolysis) is less than 3500 mg/dL(2%), the concentration of triglyceride (simulated lipid blood) is less than 3 mg/mL, testing results will not be affected.
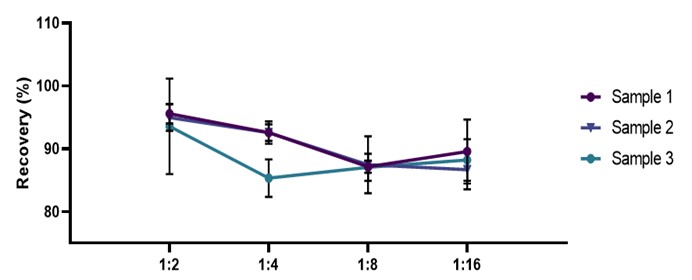
稀释线性(Dilution Linearity)
High concentrations of human IL-17A serum samples were diluted with 1:2, 1:4, 1:8 and 1:16 ratios for gradient dilution to evaluate the linearity of the assay. In the serum samples, the average detection rate of IL-17A was 90.14%.
批内差异(Intra-Assay Statistics)
Ten replicates of each of 4 samples containing different IL-17A concentrations were tested in one assay. Acceptable criteria: CV<10%.

批间差异(Inter-Assay Statistics)
Five samples containing different concentrations of IL-17A were tested in independent assays. Acceptable criteria: CV<15%.

回收率(Recovery)
Recombinant IL-17A was spiked into 3 human serum samples, and then analyzed. The average recovery of IL-17A for serum samples is 85.37%.

组分(Materials Provided)
| ID | Components | Size |
| CEA092-C01 | Pre-coated Anti-IL-17A Antibody Microplate | 1 plate |
| CEA092-C02 | Human IL-17A Standard | 20μg ×2 |
| CEA092-C03 | Biotin-Anti-IL-17A Antibody Con. Solution | 300 μL |
| CEA092-C04 | Biotin-Antibody Dilution Buffer | 8 mL |
| CEA092-C05 | Streptavidin-HRP Con. Solution | 500 uL |
| CEA092-C06 | Streptavidin-HRP Dilution Buffer | 15 mL |
| CEA092-C07 | 20× Washing Buffer | 50 mL |
| CEA092-C08 | Sample Dilution Buffer | 15 mL ×2 |
| CEA092-C09 | Substrate Solution | 12 mL |
| CEA092-C10 | Stop Solution | 6 mL |























































 膜杰作
膜杰作 Star Staining
Star Staining












