Discovery and characterization of potent broadly neutralizing antibodies from human survivors of severe fever with thrombocytopenia syndromeZhang, Shang, Han
et alEBioMedicine (2025) 111, 105481
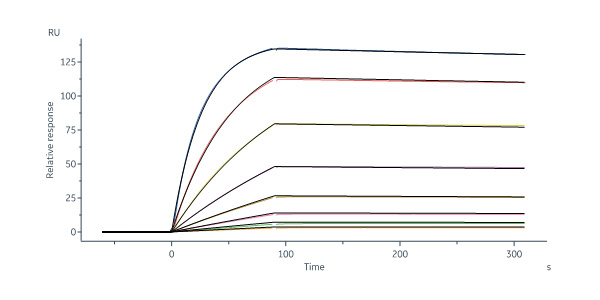
Abstract: Severe fever with thrombocytopenia syndrome virus (SFTSV) is an emerging tick-borne phlebovirus that causes viral hemorrhagic fever. Pandemic concerns have arisen due to the increased human-to-human transmission and high mortality rate, highlighting the urgent need for specific therapeutics.Our observational study characterized the memory B cell response to natural SFTSV infection in four survivors. Monoclonal antibodies (mAbs) targeting the SFTSV glycoprotein N (Gn) were isolated and tested for in vitro neutralizing activities and effects on virus binding. Structural analysis was performed to identify neutralizing epitopes recognized by the mAbs. Prophylactical and therapeutical protections were evaluated using a lethal SFTSV infection model.The selected mAbs exhibiting neutralizing activity primarily originate from the IGHV5-51 and IGHV3-30 germlines and target four distinct antigenic sites on SFTSV Gn. These elite mAbs effectively blocked the interaction between Gn and the cell receptor, preventing infections from five phylogenetically distinct SFTSV clades. Structural analysis revealed a novel neutralizing epitope located within SFTSV Gn domain I recognized by the elite mAbs. In mice of lethal infections with different SFTSV strains, administering a low dose of elite mAbs significantly improved survival rates in both prophylactic and therapeutic settings.This study identifies potent broadly neutralizing antibodies that holds promise for use in humans against SFTSV infection and highlights inhibition of receptor binding as a crucial mechanism for effective antibody-mediated neutralization against phleboviruses.The National Key Research and Development Plan of China (2018YFE0200401, 2022YFC2303300), National Natural Science Foundation of China (81825019), China Postdoctoral Science Foundation (2023M741824).Copyright © 2024 The Author(s). Published by Elsevier B.V. All rights reserved.
Molecular mechanism and structure-guided humanization of a broadly neutralizing antibody against SFTSVYang, Wu, Shang
et alPLoS Pathog (2024) 20 (9), e1012550
Abstract: Severe fever with thrombocytopenia syndrome virus (SFTSV) is a novel tick-borne bunyavirus that causes severe fever with thrombocytopenia syndrome (SFTS), with a high mortality rate of up to 30%. The envelope glycoproteins of SFTSV, glycoprotein N (Gn) and glycoprotein C (Gc), facilitate the recognition of host receptors and the process of membrane fusion, allowing the virus to enter host cells. We previously reported a monoclonal antibody, mAb 40C10, capable of neutralizing different genotypes of SFTSV and SFTSV-related viruses. However, the specific neutralization mechanism is poorly understood. In this study, we elucidated the high-resolution structure of the SFTSV Gn head domain in complex with mAb 40C10, confirming that the binding epitope in the domain I region of SFTSV Gn, and it represented that a novel binding epitope of SFTSV Gn was identified. Through in-depth structural and sequence analyses, we found that the binding sites of mAb 40C10 are relatively conserved among different genotypes of SFTSV and SFTSV-related Heartland virus and Guertu virus, elucidating the molecular mechanism underlying the broad-spectrum neutralizing activity of mAb 40C10. Furthermore, we humanized of mAb 40C10, which is originally of murine origin, to reduce its immunogenicity. The resulting nine humanized antibodies maintained potent affinity and neutralizing activity. One of the humanized antibodies exhibited neutralizing activity at picomolar IC50 values and demonstrated effective therapeutic and protective effects in a mouse infection model. These findings provide a novel target for the future development of SFTSV vaccines or drugs and establish a foundation for the research and development of antibody therapeutics for clinical applications.Copyright: © 2024 Yang et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Gn protein expressed in plants for diagnosis of severe fever with thrombocytopenia syndrome virusChang, Shimoda, Jiang
et alAppl Microbiol Biotechnol (2024) 108 (1), 303
Abstract: Severe fever with thrombocytopenia syndrome virus (SFTSV) causes the highly fatal disease in humans. To facilitate diagnosis, the native form of subunit glycoprotein (Gn), a prime target for potential vaccines and therapies, was produced in Nicotiana benthamiana using a Bamboo mosaic virus-based vector system. By fusion with secretory signal tags, SSExt, derived from the extension protein, and the (SP)10 motif, the yield of the recombinant Gn (rGn) was remarkably increased to approximately 7 mg/kg infiltrated leaves. Ultimately, an rGn-based ELISA was successfully established for the detection of SFTSV-specific antibodies in serum samples from naturally infected monkeys. As validated with the reference method, the specificity and sensitivity of rGn-ELISA were 94% and 96%, respectively. In conclusion, utilizing well-suited fusion tags facilitates rGn production and purification in substantial quantities while preserving its antigenic properties. The rGn-ELISA, characterized by its commendable sensitivity and specificity could serve as a viable alternative diagnostic method for assessing SFTSV seroprevalence. KEY POINTS: • SFTSV Gn, fused with secretory signal tags, was expressed by the BaMV-based vector. • The plant fusion tags increased expression levels and eased the purification of rGn. • The rGn-ELISA was established and validated; its specificity and sensitivity > 94%.© 2024. The Author(s).
CCR2 is a host entry receptor for severe fever with thrombocytopenia syndrome virusZhang, Peng, Wang
et alSci Adv (2023) 9 (31), eadg6856
Abstract: Severe fever with thrombocytopenia syndrome virus (SFTSV) is an emerging tick-borne bunyavirus causing a high fatality rate of up to 30%. To date, the receptor mediating SFTSV entry remained uncharacterized, hindering the understanding of disease pathogenesis. Here, C-C motif chemokine receptor 2 (CCR2) was identified as a host receptor for SFTSV based on a genome-wide CRISPR-Cas9 screen. Knockout of CCR2 substantially reduced viral binding and infection. CCR2 enhanced SFTSV binding through direct binding to SFTSV glycoprotein N (Gn), which is mediated by its N-terminal extracellular domain. Depletion of CCR2 in C57BL/6J mouse model attenuated SFTSV replication and pathogenesis. The peripheral blood primary monocytes from elderly individuals or subjects with underlying diabetes mellitus showed higher CCR2 surface expression and supported stronger binding and replication of SFTSV. Together, these data indicate that CCR2 is a host entry receptor for SFTSV infection and a novel target for developing anti-SFTSV therapeutics.























































 膜杰作
膜杰作 Star Staining
Star Staining















