14 years of rotavirus A surveillance: unusual dominance of equine-like G3P[8] genotype with DS-1-like genotype constellation after the pandemic, Belgium, 2009 to 2023Karataş, Bloemen, Cuypers
et alEuro Surveill (2025) 30 (12)
Abstract: IntroductionDespite vaccine availability, rotavirus persists as a leading cause of gastroenteritis in children younger than 5 years.AimWe aimed to evaluate temporal changes in rotavirus epidemiology in Belgium between 2009 and 2023, including the period of the COVID-19 pandemic.MethodsWe collected 8,024 rotavirus-positive stool samples throughout Belgium. For 6,352 samples, we determined the G and/or P genotypes through sequencing of the genes encoding the outer capsid proteins VP7 and VP4.ResultsBefore the COVID-19pandemic, we received on average 622 samples per rotavirus epidemiological year, which decreased to 114 and 111 samples during the two pandemic rotavirus epidemiological years, followed by a peak of 1,048 samples in the first post-pandemic year. Notably, the proportion of cases in the age group 2-5-years increased from 20.3% before to 33% after the pandemic (p < 0.001). Over the 14-year study period, the most common genotypes were G2P[4], G3P[8] and G9P[8]. Post-pandemic data show an unusually strong dominance of the equine-like G3P[8] genotype which carried a DS-1-like genotype constellation in the period 2021 to 2023. Additionally, vaccinated individuals were significantly overrepresented among patients infected with the equine-like VP7 carrying G3P[8] rotavirus compared with other genotypes, including typical human VP7 G3P[8].ConclusionDespite the presence of typical yearly genotype fluctuations, several epidemiological changes were associated with the COVID-19 pandemic, including the unusual dominance of an emerging rotavirus strain against which current vaccines may be less effective. It is essential to closely monitor this strain to determine if the phenomenon is temporary.
Identification of a potential interspecies reassortant rotavirus G and avastrovirus 2 co-infection from black-headed gull (Chroicocephalus ridibundus) in HungaryPankovics, Takáts, Urbán
et alPLoS One (2025) 20 (3), e0317400
Abstract: The black-headed gull is the most common nesting gull species in Hungary. Based on the lifestyle and feeding habits of the black-headed gull, which is highly adapted to the human environment, they can be carriers and spreaders of potential human and other animal pathogens. Between 2014 and 2018 within the framework of the "Life Bird Ringing program" a total of 7 faecal samples were collected from gulls and one sample (MR04) was randomly selected for viral metagenomics and mass sequencing. 95.4% and 4% of the reads were classified into family Seadornaviridae and Astroviridae, respectively, and then were verified by RT-PCR method. In this study, the complete genome of a potential interspecies reassortant rotavirus (RV) strain gull/MR04_RV/HUN/2014 (PP239049-PP239059) and the partial ORF1ab, complete ORF2 of a novel avian nephritis virus strain gull/MR04_AAstV/HUN/2014 (PP239060) was discussed. The strain gull/MR04_RV/HUN/2014 was closely related to rotavirus G (RVG) viruses based on the proteins VP1-VP3, VP6, NSP2, NSP3, and NSP5, but it was more related to the human rotavirus B (RVB) strain Bang373 based on the NSP1, NSP4 and VP7, VP4 proteins, which is assumed to be the result of reassortment between different RVG-RVB rotavirus species. The strain gull/MR04_AAstV/HUN/2014 belonged to the genus Avastrovirus species avastrovirus 2 (AAstV-2) and is related to members of group 6 of avian nephritis viruses (ANVs), but based on the genetic distances it may be the first representative of a separate group. Additional gull samples were found to be negative by RT-PCR. Gulls, which are well adapted to the human environment, could potentially spread enterically transmitted viral pathogens like interspecies reassortant rotaviruses (RVG/RVB), but further molecular surveillance is needed to explore more deeply the viral communities of gulls or other related species adapted to human environments.Copyright: © 2025 Pankovics et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Rotavirus Infection in Children Suffering from Diarrhea in Patna, Bihar Region, India: A Hospital-Based StudyJindal, Kumar, Bhat
et alAm J Trop Med Hyg (2025)
Abstract: Rotavirus (RV) is a leading cause of severe gastroenteritis in infants and young children, often resulting in dehydration and hospitalization. Although global data on RV is well-documented, there is limited information on its prevalence and genetic diversity in Bihar, India. This study aimed to investigate the prevalence of RV infections in the patient, from January 2021 to June 2024, and assess the associated molecular and epidemiological patterns. In this retrospective study conducted at the Rajendra Memorial Research Institute of Medical Sciences, Patna, 1,820 stool samples from patients suspected of RV infection were collected. Enzyme immunoassays were used to detect RV antigens, and positive samples were confirmed by real-time polymerase chain reaction targeting the VP4, VP6, and VP7 genes. Phylogenetic analysis was performed to examine genetic diversity. Results showed a 10% positivity rate for RV, with 5% showing equivocal results. The highest prevalence was in the 6-11 years age group (72 positive cases), followed by the 0-5 years group (62 positive cases). Prevalence decreased in older age groups, suggesting immunity through natural infection or vaccination. Phylogenetic analysis revealed distinct regional clusters and genetic variability between strains from Bihar and other parts of India, such as New Delhi and Kolkata. This study provides valuable baseline data on RV prevalence and genetic diversity in Bihar, emphasizing the need for vaccination and surveillance, particularly for younger children at higher risk. The observed genetic diversity suggests regional variations, highlighting the importance of continuous surveillance across India.
Variations in Prevalence and Characteristics of Rotavirus Diarrhea Among Outpatients - Shanghai Municipality, China, 2017-2023Gong, Kuang, Zheng
et alChina CDC Wkly (2025) 7 (7), 244-252
Abstract: This study investigated temporal changes in rotavirus group A (RVA) prevalence, epidemiological characteristics, and genotype distribution patterns among diarrhea outpatients in Shanghai Municipality, China.We conducted prospective active surveillance of diarrheal disease in pediatric and adult outpatients in Shanghai. Stool specimens were analyzed for five viral and twelve bacterial pathogens. Real-time reverse transcription polymerase chain reaction (rRT-PCR) was employed for RVA detection, followed by genotyping of RVA-positive specimens through partial amplification of VP7 and VP4 genes.The study analyzed 2,331 diarrhea cases in children aged 0-14 years and 8,418 cases in individuals aged ≥15 years between January 2017 and December 2023. Overall RVA positivity rates decreased significantly from 7.43% in 2017 to 1.19% in 2023 (P=0.024). The most pronounced decline occurred in children aged 2-5 years, where positivity rates fell from 13.08% to 1.72%. Adults aged ≥30 years also showed a substantial reduction. Among RVA-positive pediatric cases (≤14 years), the proportion of cases aged 6-14 years increased from 2.33% to 18.18%. While G9P[8] remained the predominant strain, its prevalence decreased from 77.78% to 31.25%, concurrent with the emergence of G8P[8] strains.RVA prevalence has shown a marked decline since 2018-2019, accompanied by a shift in age distribution toward older children. The diminishing dominance of G9P[8] strains coincided with the emergence of G8P[8] strains. Continued epidemiological and genetic surveillance of rotavirus diarrhea, coupled with real-world effectiveness evaluations of domestic vaccines, remains crucial for optimizing rotavirus immunization strategies.Copyright © 2025 by Chinese Center for Disease Control and Prevention.
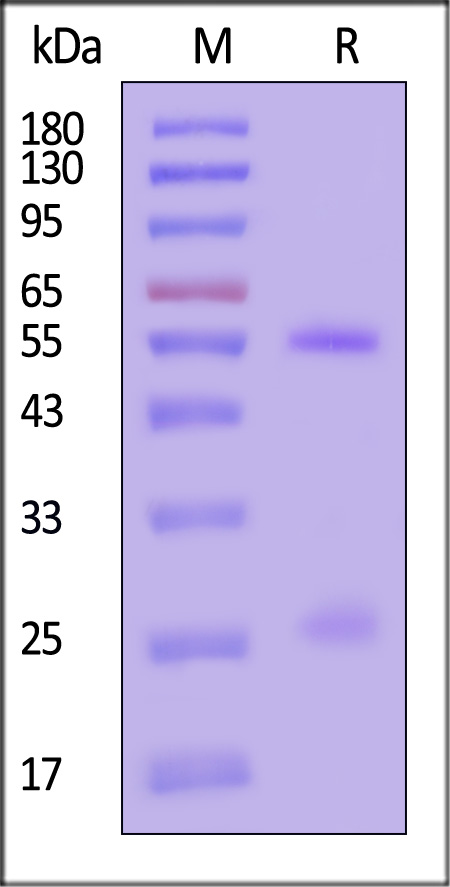
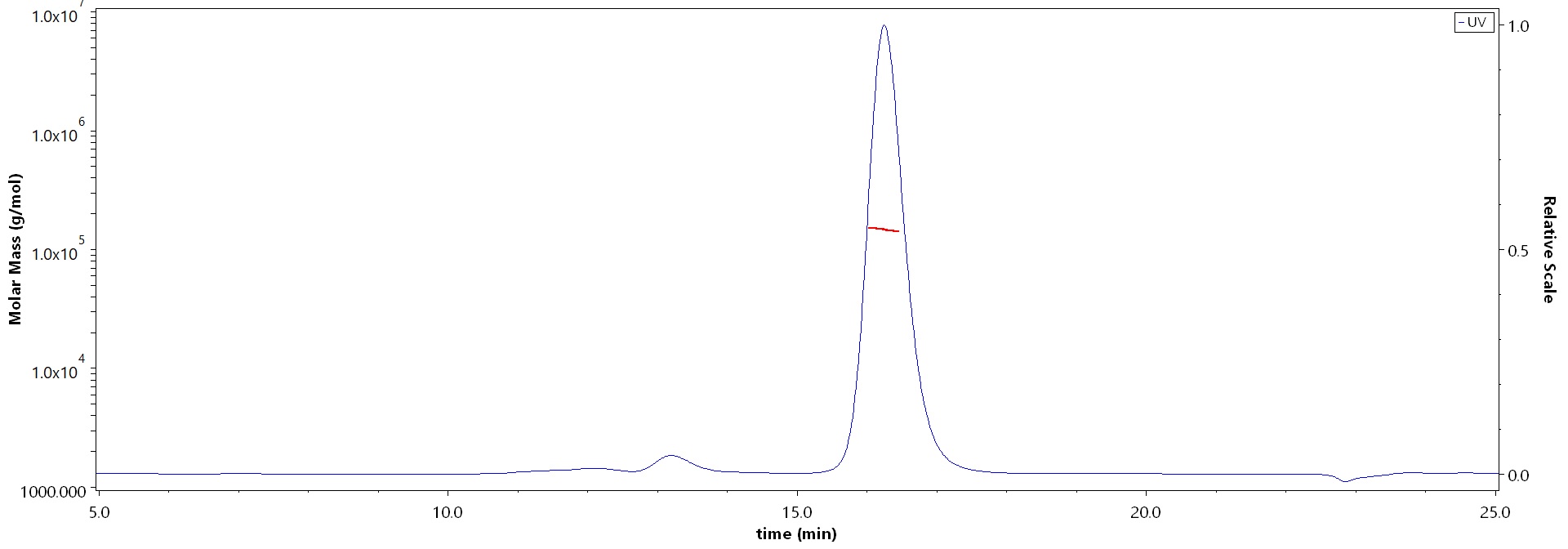
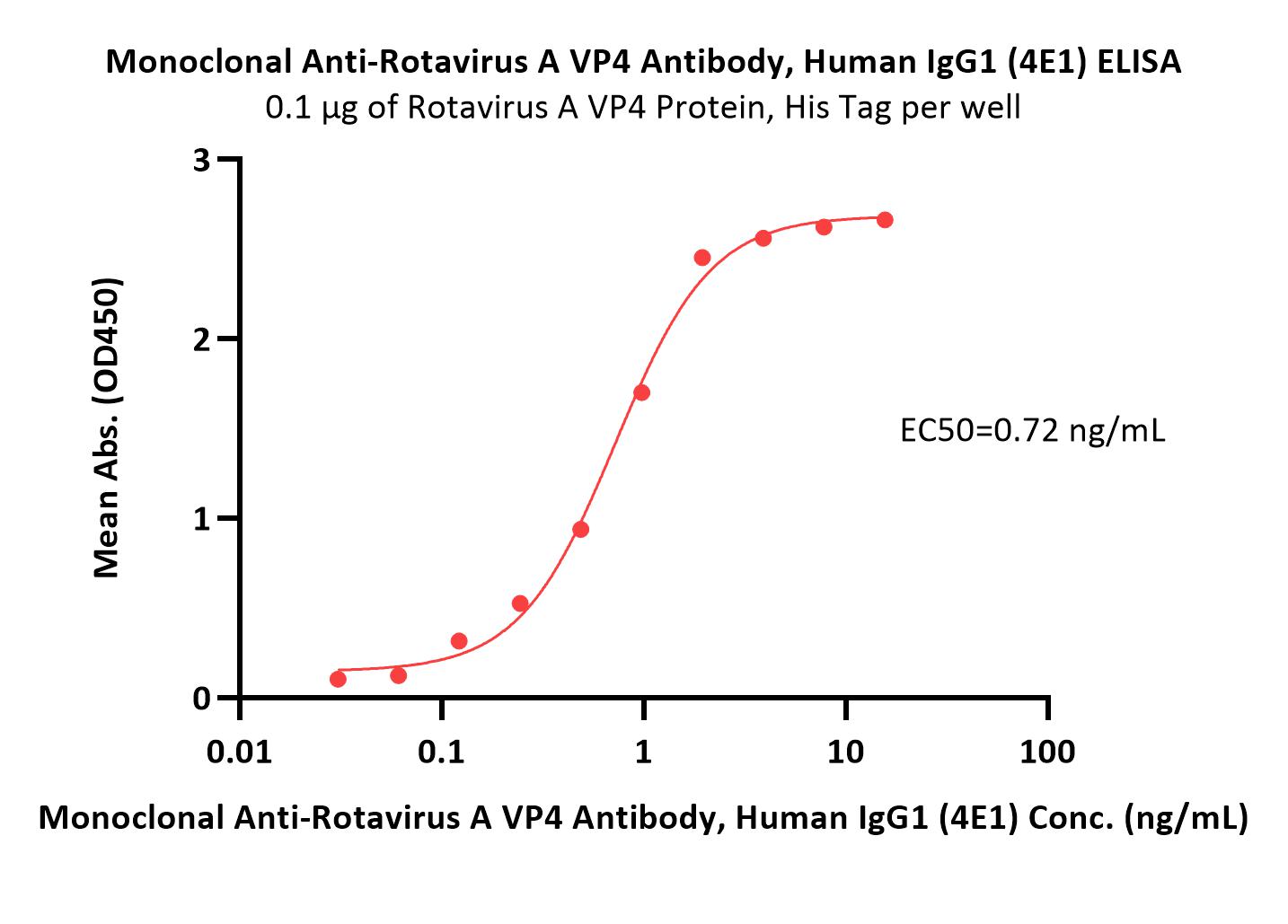
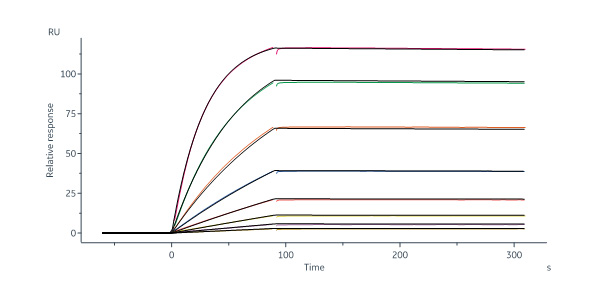























































 膜杰作
膜杰作 Star Staining
Star Staining















