- Comparable to LAL method - Endpoint fluorescent assay, comparable to other chromogenic quantitative LAL methods.
- High specificity - Unlike LAL Assay, as Factor G is absent from the rFC test kit, false-positive results due to β-glucan activation are not expected to occur.
- Accuracy - Traceability of endotoxin standards in the kit against USP Standard (Catalog No: 1235503).
- Fast time to results - 1 hours.
- High sensitivity - Sensitivity range from 0.005-5 EU/mL.
- Extensive validation – Verification on multiplex biological products, kinds of microplate readers and various buffer systems, comprehensive validation of specificity, sensitivity, precision, accuracy, applicability, and other aspects according to the parameters listed in the EUROPEAN PHARMACOPOEIA 11.0 and USP chapter<<1225>>.
- Sustainable resource - Get rid of dependence on animal derived reagents, reduce dependence on horseshoe crab resources and fishing pressure and realize long-term supply.
- Good inter batch consistency - Batch consistency of products is guaranteed due to the use of genetic recombination technology for production.
重组C蛋白:拯救鲎宝贝,共创绿色未来

原理(Assay Principles)
The Recombinant Factor C Endotoxin Detection Kit is a novel endotoxin detection method based on the recombination technology. Recombinant Factor C, as the first component of the horseshoe crab coagulation cascade reaction, is activated by an endotoxin. The activated Factor C can cleave the fluorogenic substrate and produce a fluorescent signal. The increase of fluorescence signal is positively correlated with the dosage of endotoxin. The experiment is carried on a white 96-well plate and is measured at time zero and after a one-hour 37℃ incubation. Use a fluorescence microplate reader to measure at the wavelength of ex/em = 380/440 nm to determine whether the sample is contaminated by endotoxin.
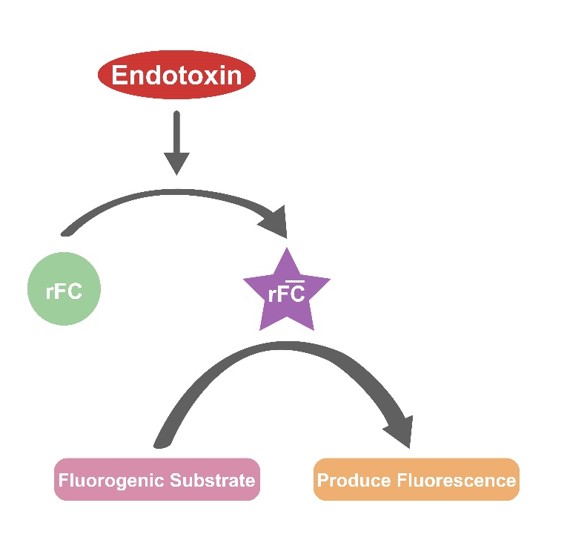
产品描述(Product Details)
| Assay Type | FRET |
| Analyte | Endotoxin |
| Format | 48T, 96T |
| Regulatory Status | RUO |
| Sensitivity | 0.005 EU/mL |
| Standard Curve Range | 0.005 EU/mL-5 EU/mL |
| Assay Time | 1 hr |
| Suitable Sample Type | For the quantitative determination of endotoxin from pharmaceutical products, biologicals for injection and some media for tissue cultures. |
| Sample volume | 100 μL |
组分(Materials Provided)
| ID | Components | Size |
| RES056-C01 | Bacterial Endotoxin Standard | 1 vial |
| RES056-C02 | Recombinant Factor C Protein | 48 tests/96 tests |
| RES056-C03 | Fluorogenic Substrate | 48 tests/96 tests |
| RES056-C04 | Water for Bacterial Endotoxins Test | 50 mL |
应用说明(Application)
Production process (Process control)
- Raw materials
- Water testing
- Intermediate product testing
Final product (Release testing)
- Parenteral drugs & Biological products
- Infusion, injection or tranfusion cells
- Cell culture media
- Medical devices
It is for research use only.
存储(Storage)
2-8℃
活性(Bioactivity)-Fluorescence Please refer to DS document for the assay protocol.
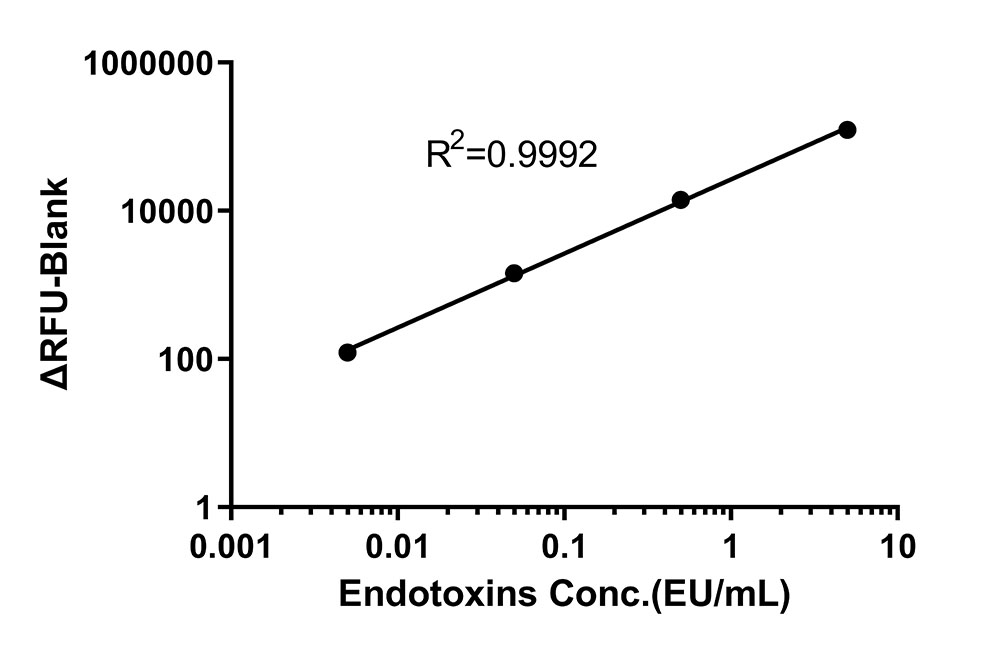
Take the logarithm of the concentration of the Endotoxin working standard solution as the abscissa, take the ΔRFU as the ordinate. Fitting the standard curve with linearly model, and the correlation coefficient R should be ≥ 0.98 (QC tested).
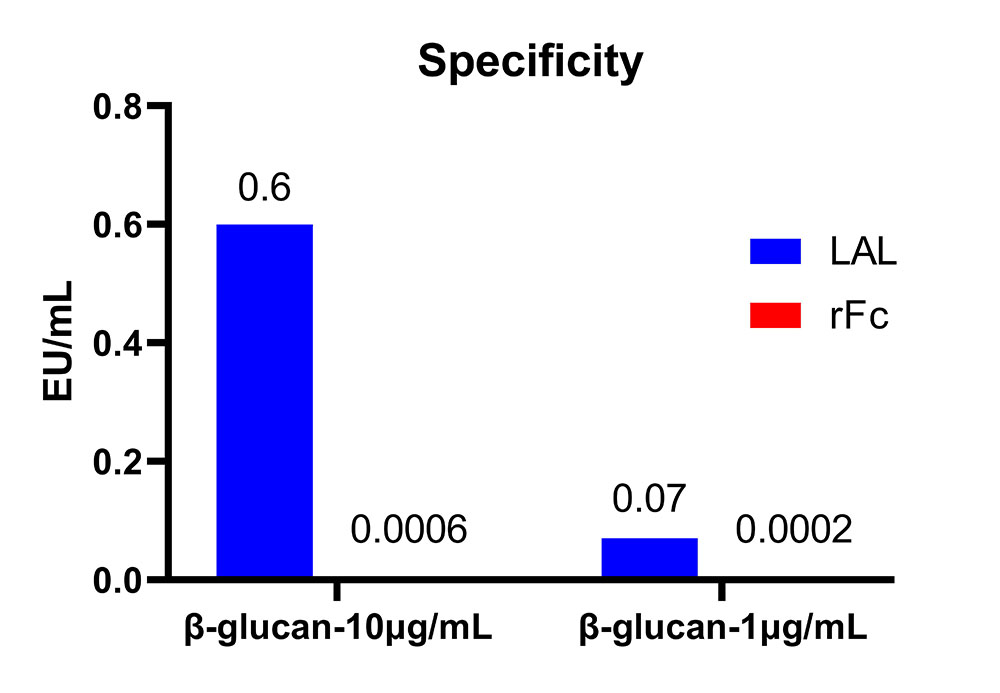
The rFC method was employed to detect endotoxin residues in β-glucan at concentrations of 10ug/mL and 1ug/mL. No non-specific signals were detected. In contrast, the dynamic chromogenic method used for β-glucan detection resulted in the detection of endotoxin and non-specific signals. This indicates that recombinant factor C does not react with β-glucan, demonstrating the good specificity of the rFC method.























































 膜杰作
膜杰作 Star Staining
Star Staining













