CRABP2 (Cellular Retinoic Acid Binding Protein 2D): A novel biomarker for the diagnosis and prognosis involved in immune infiltration of lung adenocarcinomaCai, Tian, Zhang
et alJ Cancer (2025) 16 (5), 1631-1646
Abstract: Objective: Overexpressed CRABP2 (Cellula Retinoi Aci Bindin Protei 2D) can promote progression of various tumors. However, there are few comprehensive analysis studies on CRABP2 in lung adenocarcinoma (LUAD). Methods: Several large public databases and online analysis tools such as TCGA, GEO, GEPIA2, UALCAN, Kaplan Meier plotter, LinkedOmics, TIMER, CCLE and Metascape were used for big data mining analysis. RNA interference technology, CCK8 assay, flow cytometry and apoptosis detection, and western blot were used for in vitro experiments. Results: The study revealed that the expression level of CRABP2 in plasma were higher (mean level 31.6587 ±13.8541 ng/mL vs. 13.9328 ± 5.5805 ng/mL, p<0.0001) in patients with early stage (stage IA) LUAD compared to the control group based on analysis of 640 LUAD patients and 640 matched healthy control plasma samples from Lishui Central Hospital. Receiver Operating Characteristic curve showed that CRABP2 had certain accuracy in predicting early LUAD, with a sensitivity of 70.98%, a specificity of 94.53%, a cut-off value of 0.6551 ng/mL, and an Area Under the Curve of 0.839 (95%CI: 0.817 - 0.859, p<0.0001). Compared with normal lung tissue, CRABP2 was significantly overexpressed in LUAD (p<0.05). High CRABP2 expression in LUAD predicts poor prognosis both in Overall Survival (95%CI: 1.04-1.46, HR:1.23, p=0.018) and FP (First Progression, 95%CI: 1.10-1.65, HR = 1.35, p=0.0032) in LUAD patients. CRABP2 can promote the progression of LUAD by promoting the G2/M phase transition, inhibiting the apoptosis and participating in the regulation of immune microenvironment. The high expression of CRABP2 will inhibit the recruitment of immune effector cells and promote the proportion of immuno-suppressive cells, thus promoting the progression of LUAD. The low expression of CRABP2 may enhance the expression of CD274(PD-L1), HAVCR2 and PDCD1LG2(PD-L2) in LUAD. While, the high expression of CRABP2 may enhance the expression of CTLA4, LAG3, PDCD1(PD-1), TIGIT and IGSF8 in LUAD. Conclusions: CRABP2 may be a valuable biomarker for diagnosis, treatment and prognosis of LUAD. Patients with high expression of CRABP2 in LUAD may have suboptimal efficacy when treated with inhibitors targeting CD274, HAVCR2, and PDCD1LG2, whereas they may experience better efficacy with inhibitors targeting CTLA4, LAG3, PDCD1, TIGIT, and IGSF8. Most of cancer patients with high CRABP2 expression may benefit from immune checkpoint inhibitor therapy. Our study results have laid a positive foundation for LUAD diagnosis and therapy.© The author(s).
IGSF8 impairs migration and invasion of trophoblast cells and angiogenesis in preeclampsiaChu, Chen, Guo
et alExp Cell Res (2025) 445 (1), 114405
Abstract: Insufficient trophoblast cell infiltration is implicated in the progression of preeclampsia (PE). The immunoglobulin superfamily member 8 (IGSF8) has been shown to promote cell migration, invasion, and epithelial mesenchymal transition (EMT). However, the specific impact of IGSF8 on trophoblast cells in PE has not been definitively demonstrated. To address this, placental tissues from PE patients and normal subjects was collected. A PE-like rat model was established by administering L-NAME (60 mg/kg) intragastrically to pregnant rats from the 10th to the 19th day of gestation. Knockdown and overexpression plasmids of IGSF8 were transfected into JEG-3 cells for further experiments. Clinical samples indicated impaired spiral artery remodeling, and high IGSF8 expression in the placental tissues of PE patients. PE rats exhibited increased mean arterial pressure, elevated 24-h urine protein levels, higher abortion rates, and decreased placental and fetal weight compared to rats of sham group. Failure of physiological transformation of spiral arteries was observed in PE rats, along with increased IGSF8 expression. IGSF8 overexpression inhibited JEG-3 cell migration, invasion and EMT, as well as reduced release of VEGF in JEG-3 cells, impairing HUVEC tube formation. mRNA-sequencing analysis of JEG-3 cells transfected with shIGSF8 showed differentially expressed genes related to angiogenesis, and mesenchymal cell differentiation, with IGSF8 knockdown being associated with the activation of pathways involved in blood vessel development and cell migration. Overall, this study suggests that IGSF8 plays a role in the development of PE and provides new insights for potential treatments.Copyright © 2025 Elsevier Inc. All rights reserved.
Matrix stiffness regulates the protein profile of extracellular vesicles of pancreatic cancer cell linesFerrara, Bourgoin-Voillard, Habert
et alProteomics (2024) 24 (23-24), e2400058
Abstract: The fibrotic stroma characterizing pancreatic ductal adenocarcinoma (PDAC) derives from a progressive tissue rigidification, which induces epithelial mesenchymal transition and metastatic dissemination. The aim of this study was to investigate the influence of matrix stiffness on PDAC progression by analyzing the proteome of PDAC-derived extracellular vesicles (EVs). PDAC cell lines (mPDAC and KPC) were grown on synthetic supports with a stiffness close to non-tumor (NT) or tumor tissue (T), and the protein expression levels in cell-derived EVs were analyzed by a quantitative MSE label-free mass spectrometry approach. Our analysis figured out 15 differentially expressed proteins (DEPs) in mPDAC-EVs and 20 DEPs in KPC-EVs in response to matrix rigidification. Up-regulated proteins participate to the processes of metabolism, matrix remodeling, and immune response, altogether hallmarks of PDAC progression. A multimodal network analysis revealed that the majority of DEPs are strongly related to pancreatic cancer. Interestingly, among DEPs, 11 related genes (ACTB/ANXA7/C3/IGSF8/LAMC1/LGALS3/PCD6IP/SFN/TPM3/VARS/YWHAZ) for mPDAC-EVs and 9 (ACTB/ALDH2/GAPDH/HNRNPA2B/ITGA2/NEXN/PKM/RPN1/S100A6) for KPC-EVs were significantly overexpressed in tumor tissues according to gene expression profiling interaction analysis (GEPIA). Concerning the potential clinical relevance of these data, the cluster of ACTB, ITGA2, GAPDH and PKM genes displayed an adverse effect (p < 0.05) on the overall survival of PDAC patients.© 2024 Wiley‐VCH GmbH.
IGSF8 is an innate immune checkpoint and cancer immunotherapy targetLi, Wu, Sheng
et alCell (2024) 187 (11), 2703-2716.e23
Abstract: Antigen presentation defects in tumors are prevalent mechanisms of adaptive immune evasion and resistance to cancer immunotherapy, whereas how tumors evade innate immunity is less clear. Using CRISPR screens, we discovered that IGSF8 expressed on tumors suppresses NK cell function by interacting with human KIR3DL2 and mouse Klra9 receptors on NK cells. IGSF8 is normally expressed in neuronal tissues and is not required for cell survival in vitro or in vivo. It is overexpressed and associated with low antigen presentation, low immune infiltration, and worse clinical outcomes in many tumors. An antibody that blocks IGSF8-NK receptor interaction enhances NK cell killing of malignant cells in vitro and upregulates antigen presentation, NK cell-mediated cytotoxicity, and T cell signaling in vivo. In syngeneic tumor models, anti-IGSF8 alone, or in combination with anti-PD1, inhibits tumor growth. Our results indicate that IGSF8 is an innate immune checkpoint that could be exploited as a therapeutic target.Copyright © 2024 Elsevier Inc. All rights reserved.

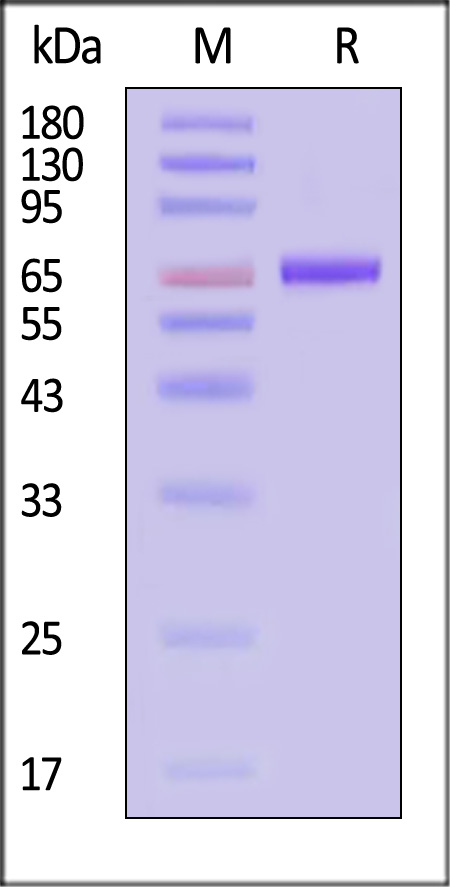
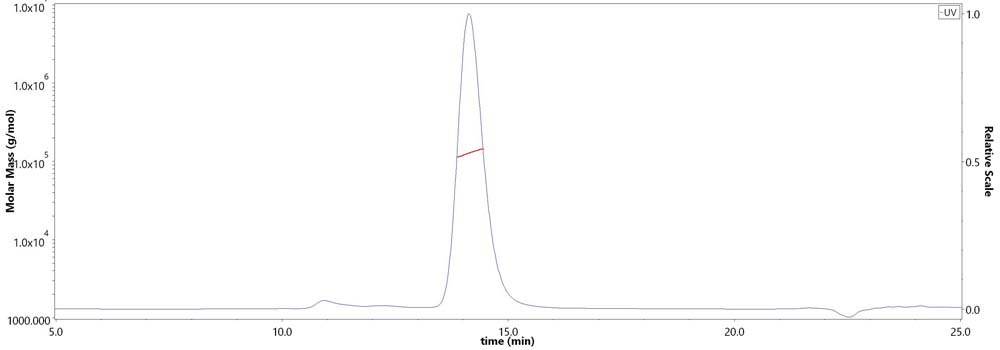























































 膜杰作
膜杰作 Star Staining
Star Staining
















