The Role of Gene Therapy as an Emerging Treatment Strategy for Alpha-1 Antitrypsin Deficiency-Associated Lung Disease: A Systematic ReviewAfrin, Binte Hasan, Sagar
et alCureus (2025) 17 (2), e79286
Abstract: Monogenetic disease alpha-1 antitrypsin deficiency (AATD) is the leading cause of emphysema, which is a major life-limiting chronic obstructive pulmonary disease (COPD). The current standard of care for severely affected individuals with lung disease is the periodic intravenous infusion of human AAT protein to restore circulating AAT levels to a protective level, known as augmentation therapy. We did a systematic review to see the effect of gene therapy as a potential therapeutic option for AATD-related lung diseases. MEDLINE (via PubMed), SCOPUS, Web of Science, Cochrane Library, and EMBASE have been searched following PICO (Population, Intervention, Comparison, Outcome) criteria. After duplication removal, abstract and title screening, full-text screening was done by two individual reviewers. Then, the data were extracted, tabulated, and analyzed. A total of 1094 articles were found in the primary search. After a comprehensive review following strict inclusion and exclusion criteria, 14 articles have been included in the review. Evidence shows the response of gene therapy depends on multiple factors, e.g., what vector is used, route of therapy administration, duration of therapy, etc. The AAV8-CASI-luc vector, delivered intratracheally (IT), achieved sustained lung transgene expression for at least 52 weeks, but 29% of mice had persistent expression up to 72 weeks, providing therapeutic AAT protein levels, reducing experimental emphysema severity in mice. Intratracheal AAV8 in mice showed the highest AAT expression in the lung, outperforming AAV9, AAV5, AAV2, and AAV2 capsid mutants, providing long-term expression up to 4 months. Intrapleural administration of AAV5-hA1AT achieved higher lung and serum A1AT levels than intramuscular delivery, with AAV5 yielding 10 times higher levels than AAV2. Gene therapy using viral vectors has a potential role in producing AAT protein, which can be beneficial for AATD-related lung diseases. Human trials are necessary to establish the effectiveness and safety of gene therapy. In conclusion, while initial studies are encouraging, more research is needed to confirm the role of gene therapy.Copyright © 2025, Afrin et al.
Transcription factor EP300 targets SIRT5 to promote autophagy of nucleus pulposus cells and attenuate intervertebral disc degenerationLiu, Huang, Xu
et alBiochim Biophys Acta Mol Cell Res (2025) 1872 (4), 119933
Abstract: Intervertebral disc degeneration (IVDD) is a prevalent spinal ailment and the leading cause of chronic low back pain. Understanding the exact pathogenesis of IVDD and developing targeted molecular drugs will be important in the future. Autophagy plays a key role in the metabolic processes and in the quality control of proteins in IVDD. However, the role of autophagy in the senescence of nucleus pulposus cell (NPC), the primary cells in the intervertebral disc responsible for maintaining the disc's structure and function, is not yet clear.Gene expression profiling data of human disc tissue were obtained from the Gene Expression Omnibus GSE15227, GSE23130, and GSE70362 datasets. Autophagy-related differentially expressed genes were identified from the Molecular Signatures Database (MSigDB) database. Weighted gene co-expression network analysis (WGCNA), receiver operating characteristic (ROC) curves, and least absolute shrinkage and selection operator (LASSO) regression identified an autophagy-related hub gene that encodes the E1A binding protein EP300 transcription factor in IVDD samples. Potential downstream target genes of EP300 were identified by bioinformatics analysis. The analysis identified sirtuin 5 (SIRT5) as a potential downstream target of EP300. Chromatin immunoprecipitation (ChIP)-qPCR, small interfering RNA (siRNA), and luciferase reporter gene assays were used to verify the interaction of EP300 and SIRT5 in vitro. For in vivo experiments, SIRT5 knockout mice and SIRT5-overexpressing adeno-associated virus serotype 5 (AAV5) were constructed to verify the effect of the EP300-SIRT5 signal axis on the progression of IVDD.EP300 expression was reduced in the IVDD samples compared with its expression in healthy disc tissue samples. The reduced EP300 expression inhibited the occurrence of autophagy, which promoted NPC senescence. ChIP-qPCR and luciferase reporter gene assays showed that EP300 promoted SIRT5 expression by direct binding to its promoter. Activation of EP300 expression increased SIRT5 expression and significantly improved autophagy for inhibition of NPC senescence. In vivo experiments confirmed that knockdown of EP300 promoted NPC senescence and led to an exacerbation of IVDD, which was reversed by SIRT5 overexpression.Our results provide the first evidence for the importance of EP300 and SIRT5 interactions in promoting IVDD development by inhibiting autophagy during IVDD. The EP300-SIRT5 signaling axis was identified as a promising target for therapy of IVDD based on autophagy genes.Copyright © 2025. Published by Elsevier B.V.
Development and Validation of AAV-Mediated Liver, Liver-VAT, and Liver-Brain SORT and Therapeutic Regulation of FASN in Hepatic De Novo LipogenesisBhadury, Athar, Mishra
et alCells (2025) 14 (5)
Abstract: Hepatic lipogenesis combined with elevated endoplasmic reticulum (ER) stress is central to non-alcoholic steatohepatitis (NASH). However, the therapeutic targeting of key molecules is considerably less accomplished. Adeno-associated virus (AAV)-mediated gene therapies offer a new solution for various human ailments. Comprehensive bio-functional validation studies are essential to assess the impact of AAVs in the target organ for developing both preclinical and clinical gene therapy programs. Here, we have established a robust and efficient protocol for high-titer AAV production to enable detailed Selective ORgan Targeting (SORT) of AAV1, 5, 7, and 8 in vivo. Our results for in vivo SORT showed single organ (liver) targeting by AAV8, no organ targeting by AAV1, and dual organ transduction (liver-brain and liver-VAT) by AAV5 and AAV7. Using a human dataset and preclinical murine models of NASH, we identified an inverse correlation between ER stress-triggered CRELD2 and the de novo lipogenesis driver FASN. Furthermore, liver-specific silencing of CRELD2 via AAV8-shCreld2 strongly supports the contribution of CRELD2 to de novo lipogenesis through FASN regulation. Thus, our study demonstrates a robust method for producing clinically translatable AAVs that could be readily adapted for liver and/or liver-VAT or liver-brain targeted gene therapy.
Systemic delivery of AAV5, AAV8, and AAV9 packaging a C5-12-microdystrophin-FLAG expression cassette in non-human primatesLiu, Cook, Dai
et alMol Ther Methods Clin Dev (2025) 33 (1), 101411
Abstract: Safely achieving therapeutic expression levels with adeno-associated virus (AAV) gene therapy is a significant challenge for treating the large muscle mass in humans. Non-human primates (NHPs) provide a more accurate assessment of the feasibility of achieving an effective and safe dose than rodents. Here, we compared a single systemic administration of AAV5, AAV8, or AAV9 in NHPs, each packaging the C5-12-microdystrophin-FLAG expression cassette. At 1 month post-dose, we compared tissue vector genomes, mRNA, and microdystrophin-FLAG protein levels by meso-scale discovery-enzyme-linked immunosorbent assay, liquid chromatography-mass spectrometry, and immunofluorescence. The C5-12 promoter was highly selective for heart and skeletal muscles, when compared to off-target tissues such as peripheral blood mononuclear cells, lung, liver, and kidney. AAV8 led to higher levels of microdystrophin-FLAG mRNA and protein in the cardiac ventricles and skeletal muscles when compared to AAV5 or AAV9. The AAV8-microdystrophin-FLAG led to ∼20% of wild-type NHP dystrophin protein expression levels and was located on the sarcolemma of ∼40% of skeletal muscles fibers and ∼15% of left ventricular cardiomyocytes. Hematology, serum chemistry, and pathology were unremarkable. Thus, a systemic dose of ∼1.18 × 1014 vector genomes/kg AAV8 is predicted to be safe and efficacious for treating Duchenne muscular dystrophy (DMD) but has significant room for improvement.© 2025 Published by Elsevier Inc. on behalf of The American Society of Gene and Cell Therapy.
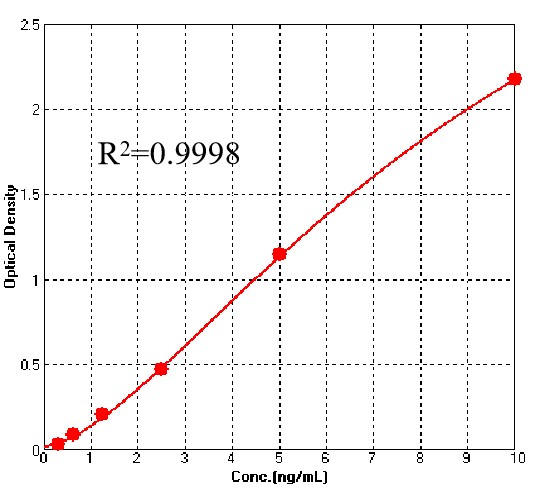
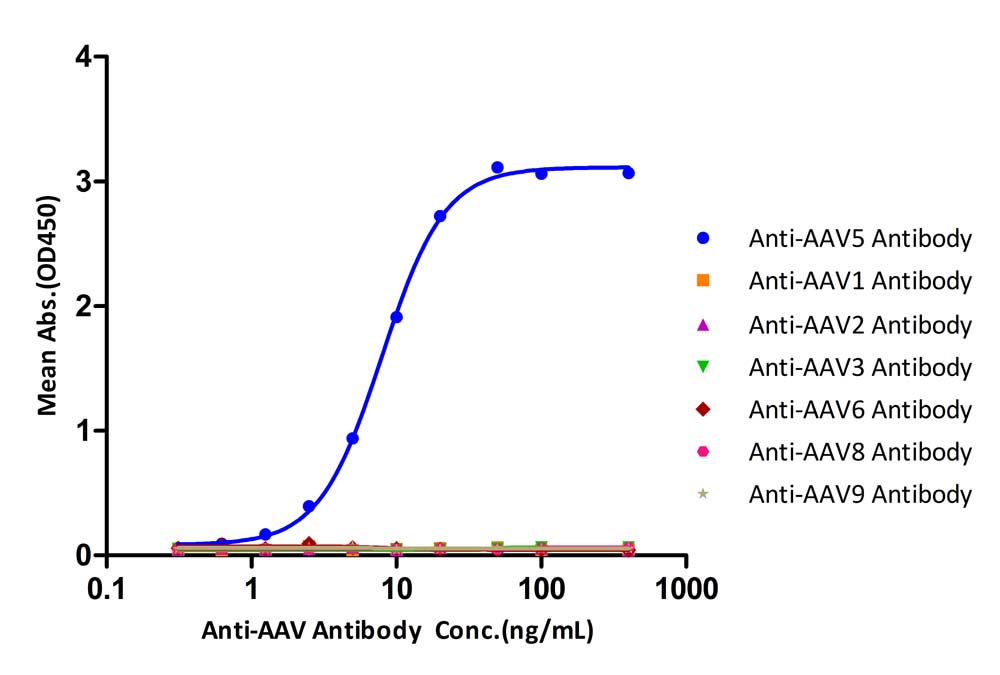
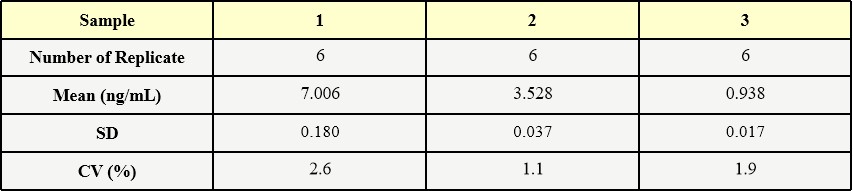
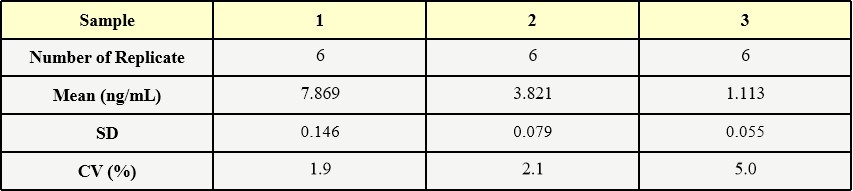























































 膜杰作
膜杰作 Star Staining
Star Staining
















