组分(Materials Provided)
| ID | Components | Size |
| RAS202-C01 | Pre-coated Anti-RSV-Pre-Fusion glycoprotein F0 (site V) Antibody Microplate | 1 plate |
| RAS202-C02 | Pre-Fusion glycoprotein F0 (RSV) Standard | 15 μg |
| RAS202-C03 | HRP-Anti-Pre-Fusion glycoprotein F0 (RSV) Antibody | 20 μg |
| RAS202-C04 | 10×Washing Buffer | 50 mL |
| RAS202-C05 | 2×Dilution Buffer | 50 mL |
| RAS202-C06 | Substrate Solution | 12 mL |
| RAS202-C07 | Stop Solution | 7 mL |
产品概述(Product Overview)
Respiratory syncytial virus (RSV) is a highly contagious virus causing severe infection in infants and the elderly. Various approaches are being used to develop an effective RSV vaccine. The RSV fusion (F) subunit, particularly the cleaved trimeric pre-fusion F, is one of the most promising vaccine candidates under development.
A rapid and effective assay kit detecting the levels of HRSV Pre-Fusion glycoprotein F0 is urgently needed to accelerate the development of RSV vaccines.
应用说明(Application)
The kit has been tested to specifically identify HRSV (A) and HRSV (B) Pre-Fusion glycoprotein. It was developed for the specific quantitative detection of HRSV Pre-Fusion glycoprotein F0 (site V) in samples.
It is for research use only.
重构方法(Reconstitution)
Please see Certificate of Analysis for details of reconstitution instruction and specific concentration.
存储(Storage)
2-8℃
质量管理控制体系(QMS)
典型数据-Typical Data Please refer to DS document for the assay protocol.
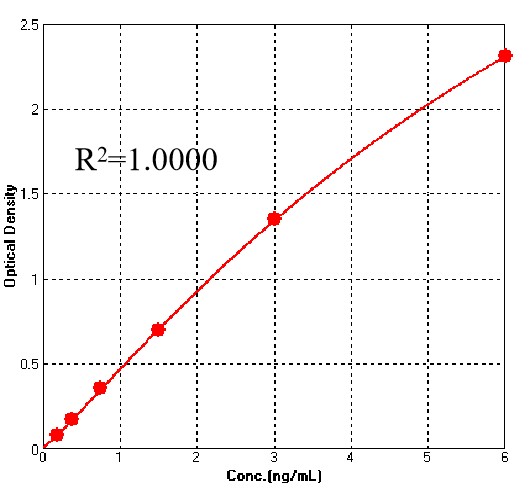
For each experiment, a standard curve needs to be set for each micro-plate, and the specific OD value may vary depending on different laboratories, testers, or equipments. The following example data is for reference only.























































 膜杰作
膜杰作 Star Staining
Star Staining















