Evaluation of hepatitis A virus recombinant proteins for detecting anti-HAV IgM and IgG antibodiesHunderkar, Ganorkar, Walimbe
et alMicrobiol Spectr (2025)
Abstract: Hepatitis A virus (HAV) is a major causative agent of self-limiting liver infections. India was highly endemic for HAV in the past; children were exposed to the virus at an early age without any disease symptoms and developed lifelong immunity. With improvements in living conditions, an epidemiological transition is occurring. There is a significant increase in hepatitis A outbreaks involving adolescents and young adults. The gold standard for hepatitis A diagnosis is anti-HAV IgM antibodies. Although antibody responses are primarily targeted against HAV structural proteins (capsid proteins), non-structural proteins are also immunogenic. In the present study, we expressed HAV capsid proteins VP1-2A, VP0 (VP4 + VP2), VP3, and non-structural protein 3CPro in the bacterial system and explored the possible use of these as antigens to detect anti-HAV IgM and IgG antibodies using a well-defined serum sample panel. The capsid protein-based assays showed overall less sensitivity for detection of both anti-HAV IgM and IgG antibodies as compared to whole virus antigen-based commercial assays. Among capsid proteins, rVP1-2A showed the highest sensitivity (86.3%) and specificity (84.2%) in detecting anti-HAV IgG, while rVP0 (VP2 + VP4) exhibited the highest sensitivity (79.5%) and specificity (80.2%) for IgM antibodies. Interestingly, r3CPro exhibited higher sensitivity (96.9%) and specificity (93.2%) in IgM detection and 93.94% sensitivity and 88% specificity for IgG, indicating its usefulness in detecting both anti-HAV IgM and IgG antibodies during the acute phase of the disease. Though 3CPro appeared to be useful in differentiating antibody responses due to infection and vaccination, our analysis revealed that the anti-3CPro antibody response is short-lived after natural infection, and hence, it cannot be used as a marker to differentiate between infection and vaccination. However, 3CPro would be useful for developing a hepatitis A diagnostic assay.Hepatitis A was highly endemic in India earlier. With recent developments, there is a shift in the endemicity to intermediate levels. This has resulted in the occurrence of hepatitis outbreaks with symptomatic infections in adolescents and adults. Occasionally, the disease manifestations are serious, leading to acute liver failure. In such a situation, there is a need for a timely diagnosis of the infection.
Isolation and molecular characteristics of D2 genotype of Aichivirus D in dairy cattle in ChinaYan, Xu, Yue
et alFront Vet Sci (2025) 12, 1551420
Abstract: Aichivirus D (AiV-D), a newly emerging member of the Kobuvirus genus, is associated with diarrhea in cattle. This study aimed to investigate the prevalence and molecular characteristics of AiV-D among dairy cattle in China. From October 2021 to August 2022, 279 fecal samples were collected from diarrheal dairy cattle across seven provinces in China. Among these, 37 samples (13.2%) tested positive for AiV-D by RT-PCR, indicating a wide geographical distribution of AiV-D in Chinese dairy cattle. Phylogenetic analysis based on the complete VP1 gene revealed that Chinese dairy cattle AiV-D strains belong to the AiV-D2 genotype, with unique amino acid changes in VP0, VP3, and VP1 that distinguish them from known AiV-D strains. Additionally, an AiV-D strain was successfully isolated, and its complete genome was sequenced. Phylogenetic analysis of the complete genome and individual genes confirmed the strain's classification within the AiV-D2 genotype. This study reports the first detection of the AiV-D2 genotype outside Japan, highlighting the need for future surveillance to better understand the epidemiology and diversity of AiV-D in China.Copyright © 2025 Yan, Xu, Yue and Tang.
Foot-and-mouth disease virus-like particle vaccine incorporating dominant T and B cell epitopes: enhanced immune response in piglets with CD154 moleculesLi, Zeng, Niu
et alFront Vet Sci (2025) 12, 1540102
Abstract: Foot-and-mouth disease (FMD) is a highly contagious disease caused by FMDV, resulting in vesicular lesions in cloven-hoofed animals and posing significant economic threats to the livestock industry. VLP vaccines, which lack viral genetic material and are non-infectious, demonstrate superior safety compared to traditional inactivated vaccines. This study employs ADDomer, a novel adenovirus-based VLP framework, to display FMDV antigenic epitopes on the VLP surface. Additionally, FMDV capsid proteins can assemble into VLPs, offering innovative approaches for developing more efficient and safer FMDV vaccines.Two FMDV VLP proteins were constructed using a baculovirus expression system. One VLP was developed by embedding the B-cell epitope of FMDV VP1 into the G-H loop of VP3 and co-expressing it with VP1 and VP0 to form VP1-VP3B-VP0. The other VLP, ADDomer-BBT, fused B-and T-cell epitopes from FMDV O-type VP1 into the ADDomer platform, with porcine CD154 expressed as an immune enhancer. Expression conditions were optimized, and proteins were purified. The VLPs, combined with porcine CD15 molecular adjuvant, were evaluated for immunogenicity in piglets.After purification, both VLPs displayed virus-like structures under electron microscopy. Immunization in piglets induced high levels of FMDV-specific and neutralizing antibodies, enhanced cytokines IL-2, IL-4, and IFN-γ, and increased lymphocyte proliferation. The CD154-added group showed higher immune responses.The VLP vaccines effectively induced strong cellular and humoral immune responses, with CD154 enhancing efficacy. These findings provide insights for developing safer, more effective FMDV vaccines and contribute to advancing livestock health and productivity.Copyright © 2025 Li, Zeng, Niu, Yuan, Li, Lin, Xie, Zhu, Yi, Ding, Zhao, Fan and Chen.
The Development of a One-Step PCR Assay for Rapid Detection of an Attenuated Vaccine Strain of Duck Hepatitis Virus Type 3 in KoreaYu, Park, Kim
et alVet Sci (2024) 12 (1)
Abstract: Duck hepatitis A virus type 3 (DHAV-3) is a viral pathogen that causes acute, high-mortality hepatitis in ducklings, and vaccination with attenuated live vaccines is currently the main preventive measure against it. However, differentiating infected from vaccinated animals (DIVA) is crucial for clinical diagnosis and effective disease control. This study aimed to develop a rapid mismatch amplification mutation assay PCR (MAMA-PCR) diagnostic method to simultaneously detect and differentiate between wild-type and vaccine strains. The method was specifically designed to target the critical single-nucleotide polymorphism (SNP) site (T→C at position 1143 in the VP0 gene) unique to the Korean vaccine strain AP04203-P100. MAMA-PCR demonstrated high sensitivity and specificity, with detection limits as low as 102.4 ELD50/mL for wild strains and 100.5 ELD50/mL for vaccine strains, and showed no cross-reactivity with 11 other common duck pathogens. The clinical sample results were completely consistent with those obtained using nested PCR detection and gold-standard sequencing. In summary, we successfully developed a rapid, one-step MAMA-PCR method that is more suitable for clinical diagnosis than traditional sequencing methods.
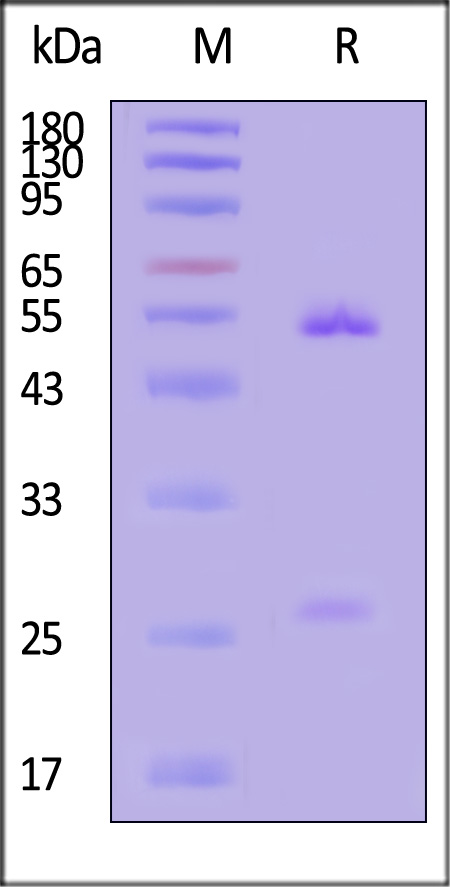
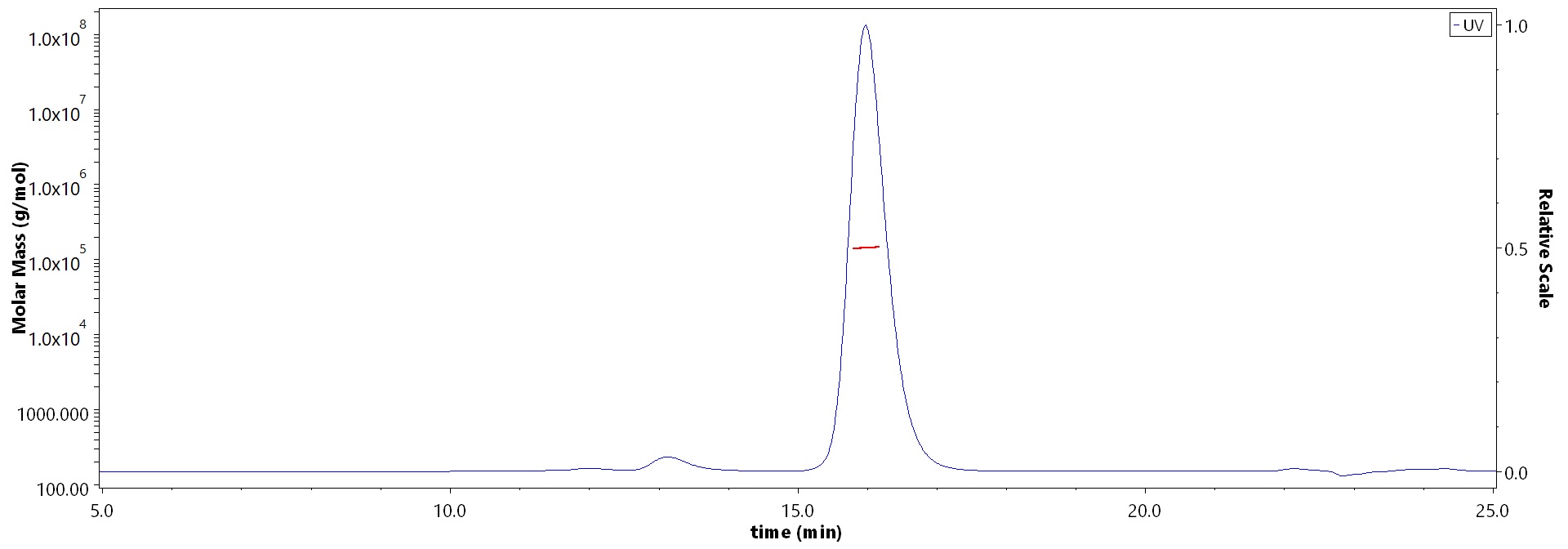
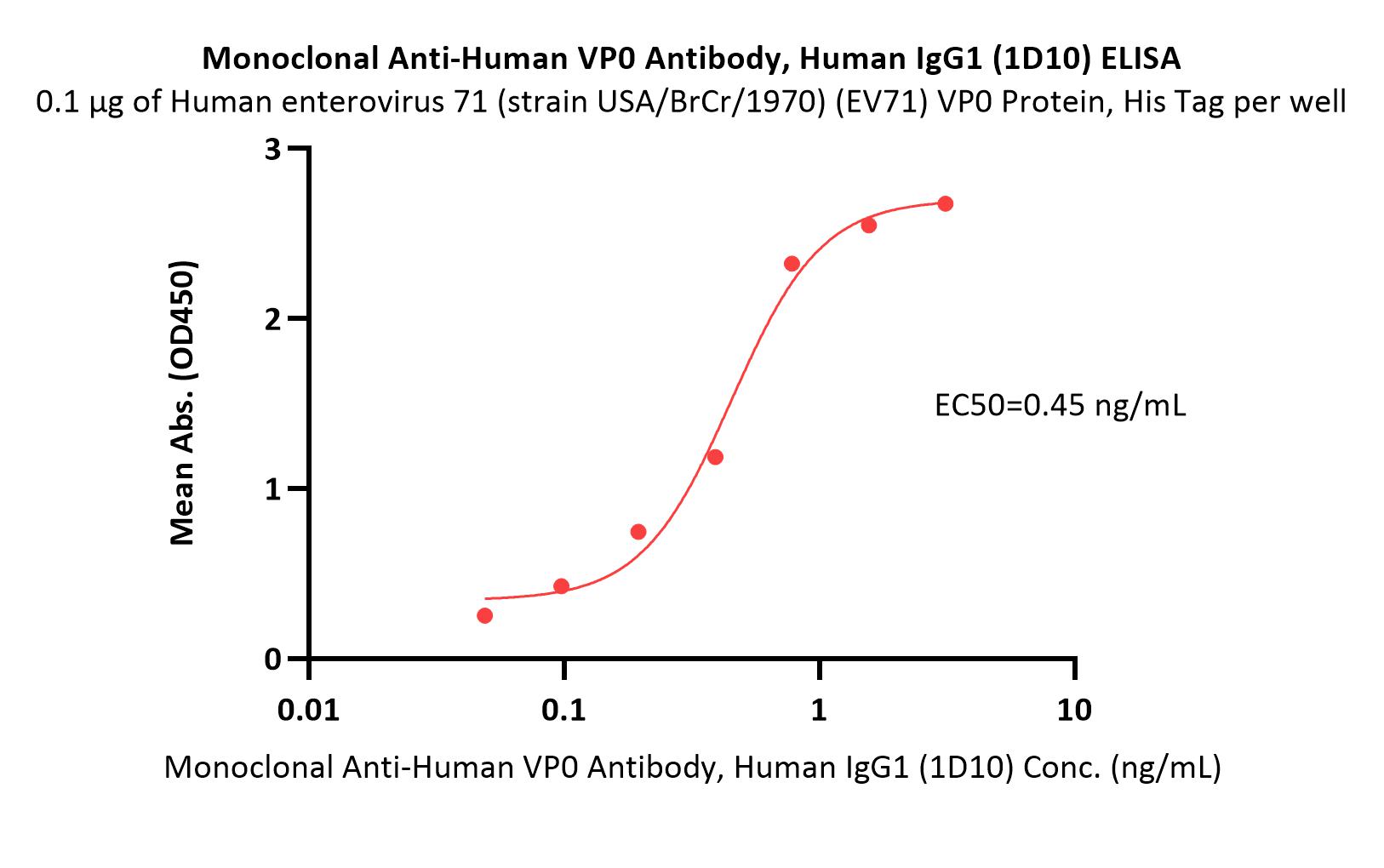
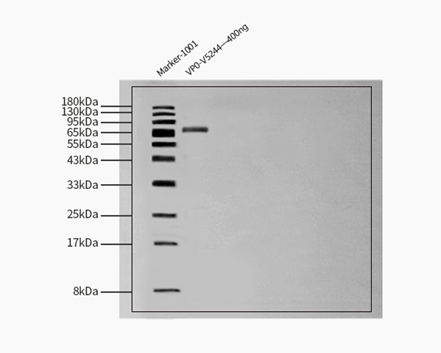























































 膜杰作
膜杰作 Star Staining
Star Staining















