Human respiratory syncytial virus genetic diversity and lineage replacement in Ireland pre- and post-COVID-19 pandemicRice, Gonzalez, Carr
et alMicrob Genom (2025) 11 (3)
Abstract: Human respiratory syncytial virus (HRSV) is a common cause of lower respiratory tract infections globally, and changes in viral epidemiology have been observed in many jurisdictions following the coronavirus disease 2019 (COVID-19) pandemic. Newly licensed vaccines and monoclonal antibodies are anticipated to alleviate the burden on healthcare systems, though such interventions may exert selective pressures on viral evolution. To evaluate the diversity of HRSV in Ireland pre- and post-COVID-19 pandemic, whole-genome sequencing was performed on HRSV-A (n=123) and -B (n=110) samples collected from community and hospitalized cases, during three HRSV seasons between 2021 and 2024. Additionally, G gene sequences, from HRSV-A (n=141) and -B (n=141), collected in the 2015-2019 period were examined. Lineages were assigned by phylogenetic analyses including reference lineages. Phylogenetic trees inferred with the G gene and whole genomes were consistent. Changes in the prevalence of certain lineages post-COVID-19 reflected the impact of non-pharmaceutical interventions (NPIs) introduced to reduce severe acute respiratory syndrome coronavirus 2 transmission, with A.D.1 and A.D.5 the dominant HRSV-A lineages and B.D.E.1 the most prevalent HRSV-B lineage. Similar trends were observed in HRSV lineages circulating across Europe during this time. The emergence of a new lineage was identified as a descendant from A.D.1, with eight distinctive substitutions in proteins G, F and L. Other circulating lineages with aa substitutions were observed in the F glycoprotein, which could impact nirsevimab binding. We provide the first comprehensive analysis of HRSV genomic diversity and evolution in Ireland over the last decade and the impact of the NPIs introduced during the COVID-19 pandemic. This study provides a foundation for future public health surveillance employing pathogen genomics to enable an evidence-based assessment of the impact of pharmaceutical interventions on HRSV evolution and disease severity.
Development of a genetically modified full-length human respiratory syncytial virus preF protein vaccineLao, Feng, Wu
et alVaccine (2025) 49, 126799
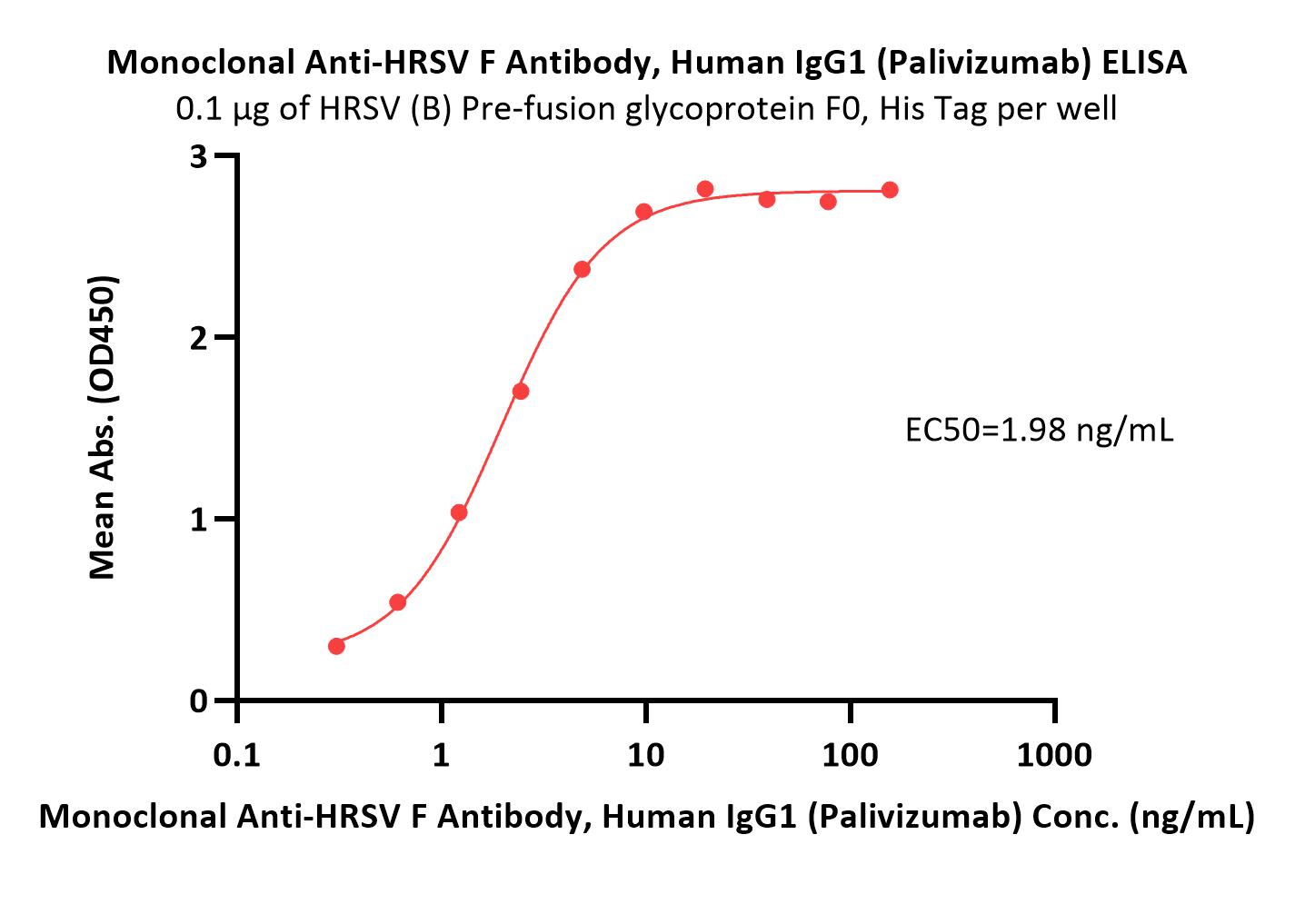
Abstract: Human Respiratory Syncytial Virus (hRSV) is a major cause of acute lower respiratory tract infections (ALRTI) in infants, the elderly, and immunocompromised individuals. The recent approval of recombinant protein-based hRSV vaccines represents significant progress in combating hRSV. However, these vaccines utilized optimized preF ectodomain attached with an exogenous trimeric motif, which may induce immunological complications. Our research addresses these concerns by employing modified "full-length" preF proteins, preF-TMCT, designed to mimic the natural F protein structure and avoid potential immunological complications. We characterized a group of preF constructs and identified two candidates that exhibited desirable expression levels, high antigenicity and good stability. Immunization of Balb/c mice confirmed the robust immunogenicity and effective in induction of cross-reactive neutralizing antibodies of these antigens, particularly the lead-construct BR40. This investigation aims to contribute new insights to hRSV vaccine development. The near-native structure of the "full-length" preF-TMCT antigen also makes it valuable for producing therapeutic monoclonal antibodies (mAbs) and other biopharmaceuticals against hRSV infection.Copyright © 2025 Elsevier Ltd. All rights reserved.
Robust and Long-Lasting Immunity and Protection in Mice Induced by Lipopolyplex-Delivered mRNA Vaccines Expressing the Prefusion Protein of Respiratory Syncytial VirusShan, Han, Cheng
et alVaccines (Basel) (2025) 13 (1)
Abstract: Respiratory syncytial virus (RSV) is the leading cause of lower respiratory tract infections in infants and children. mRNA vaccines based on the lipopolyplex (LPP) platform have been previously reported, but they remain unapplied in RSV vaccine development. In this study, we developed a novel LPP-delivered mRNA vaccine that expresses the respiratory syncytial virus prefusion protein (RSV pre-F) to evaluate its immunogenicity and protective effect in a mouse model. We synthesized mRNAs with gene modification for RSV pre-F and prepared mRNA vaccines using the LPP delivery platform, referred to as RSV pre-F LPP-mRNA. RSV pre-F protein expression in mRNA vaccines was characterized in vitro. Then, we evaluated the effects of the immune response and protection of this mRNA vaccine in mice up to 24 weeks post-vaccination. Following booster immunization, robust and long-lasting RSV pre-F-specific IgG antibodies were detected in the serum of mice, which exhibited Th1/Th2 balanced IgG response and cross-neutralizing antibodies against different subtypes (RSV A2, B18537, and clinical isolate hRSV/C-Tan/BJ 202301), with a clear dose-response relationship observed. RSV pre-F-specific IgG antibodies were maintained in the mice for an extended period, lasting up to 18 weeks post-immunization. Concurrently, multifunctional RSV F-specific CD8+ T cells (IFN-γ, IL-2, and TNF-α) were detected in the mice. After RSV A2 challenge, the RSV pre-F LPP-mRNA vaccine led to a significant reduction in viral replication, while reduced pathological damage was observed in lung tissue. The LPP-delivered mRNA vaccine expressing RSV pre-F induces a robust and long-lasting immune response and protection, indicating good prospects for further development and application.
Preliminary Study on Type I Interferon as a Mucosal Adjuvant for Human Respiratory Syncytial Virus F ProteinHu, Zhang, Cao
et alVaccines (Basel) (2024) 12 (11)
Abstract: Background: Human respiratory syncytial virus (HRSV) imposes a significant disease burden on infants and the elderly. Intranasal immunization using attenuated live vaccines and certain vector vaccines against HRSV has completed phase II clinical trials with good safety and efficacy.Recombinant protein vaccines for mucosal immunization require potent mucosal adjuvants. Type I interferon (IFN), as a natural mucosal adjuvant, significantly enhances antigen-presenting cell processing and antigen presentation, promoting the production of T and B cells. Methods: This study utilized human α2b interferon (IFN-human) and mouse α2 interferon (IFN-mouse) as nasal mucosal adjuvants in combination with fusion protein (F). Intranasal immunization was performed on BALB/c mice to evaluate the immunogenicity of the formulation in vivo. Results: Compared to the F protein immunization group, mice in the F + IFN-Human and F + IFN-Mouse experimental groups exhibited significantly increased neutralizing antibody titers and augmented secretion of IFN-γ and IL-4 by lymphocytes, and both of them could induce the production of high-titer specific IgA antibodies in mice (p < 0.001).The F + IFN-Human immunization induced the highest IgG and IgG1 antibody titers in mice; however, the F + IFN-Mouse immunization group elicited the highest neutralizing antibody titers (598), lowest viral loads in the lungs (Ct value of 31), and fastest weight recovery in mice. Moreover, mice in the F + IFN-Mouse immunization group displayed the mildest lung pathological damage (Total score of pathological injury was 2). Conclusions: In conclusion, IFN-Mouse, as a mucosal adjuvant for HRSV recombinant protein vaccines, demonstrated superior protective effects in mice compared to IFN-Human adjuvants.
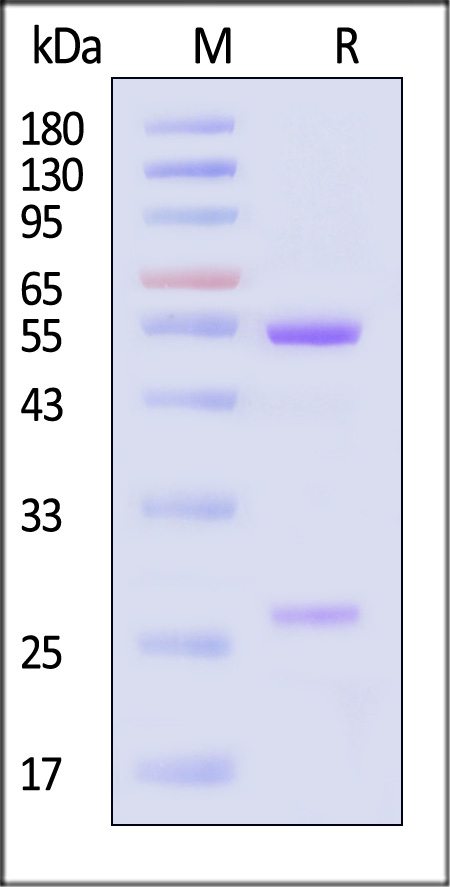
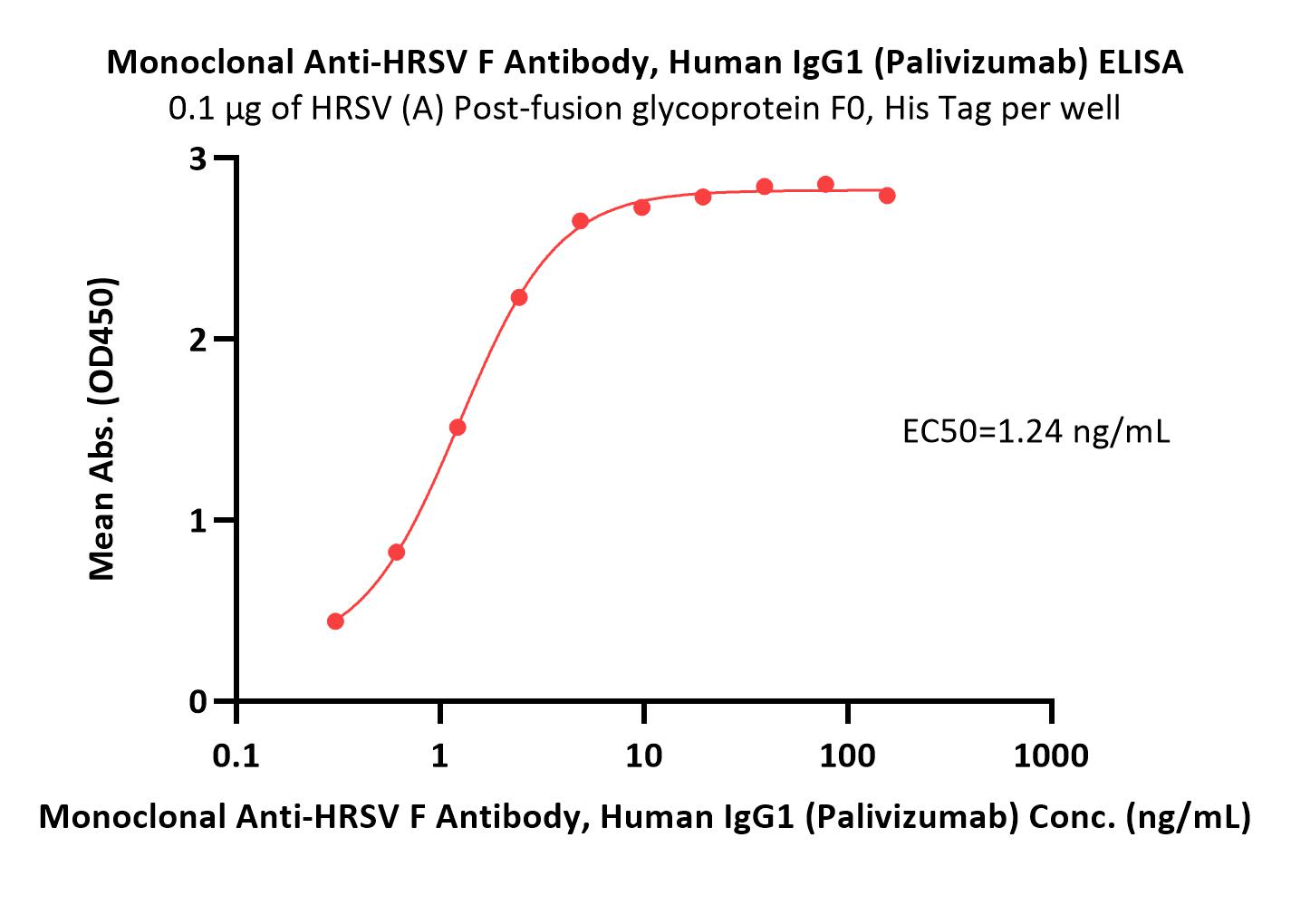
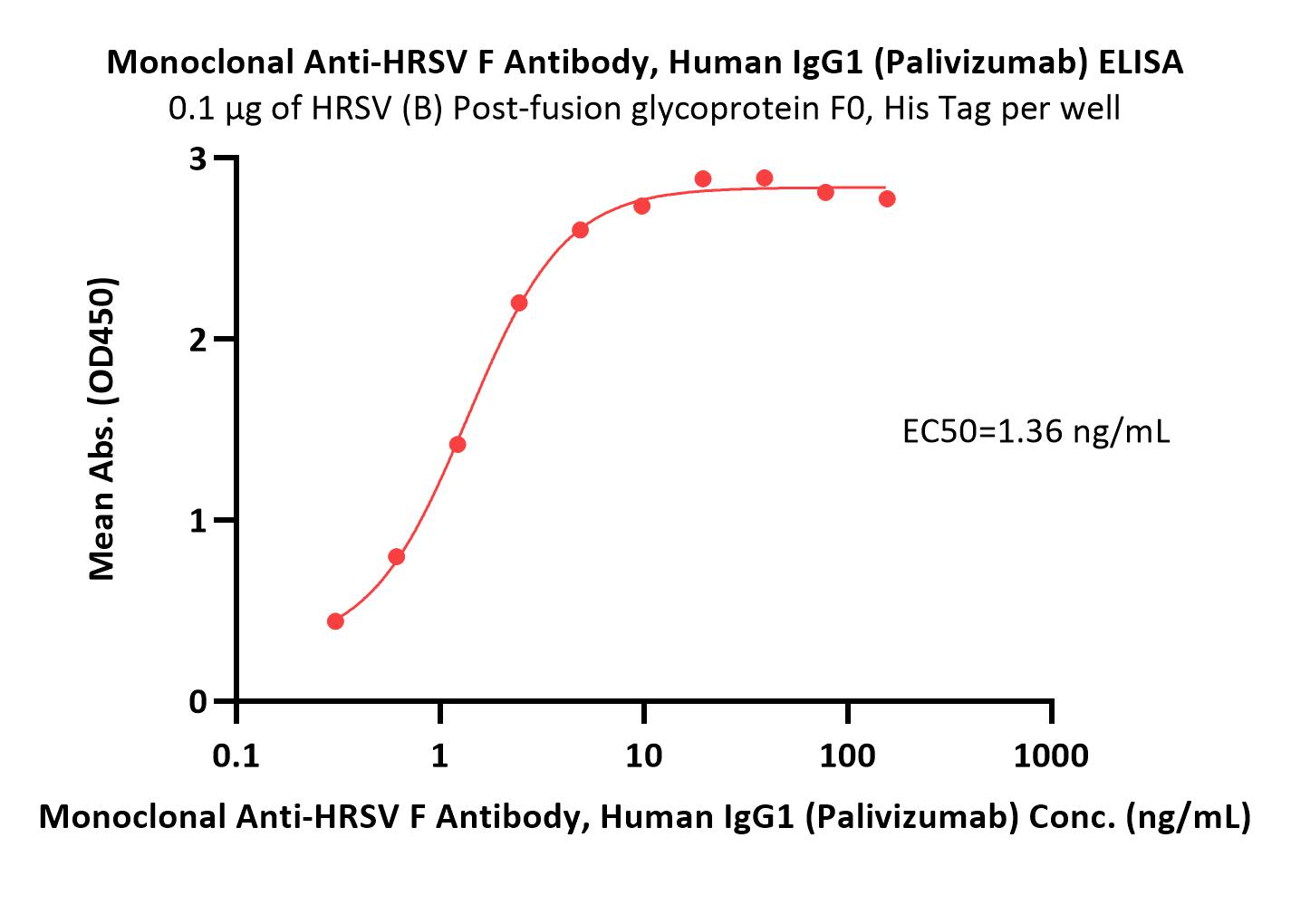
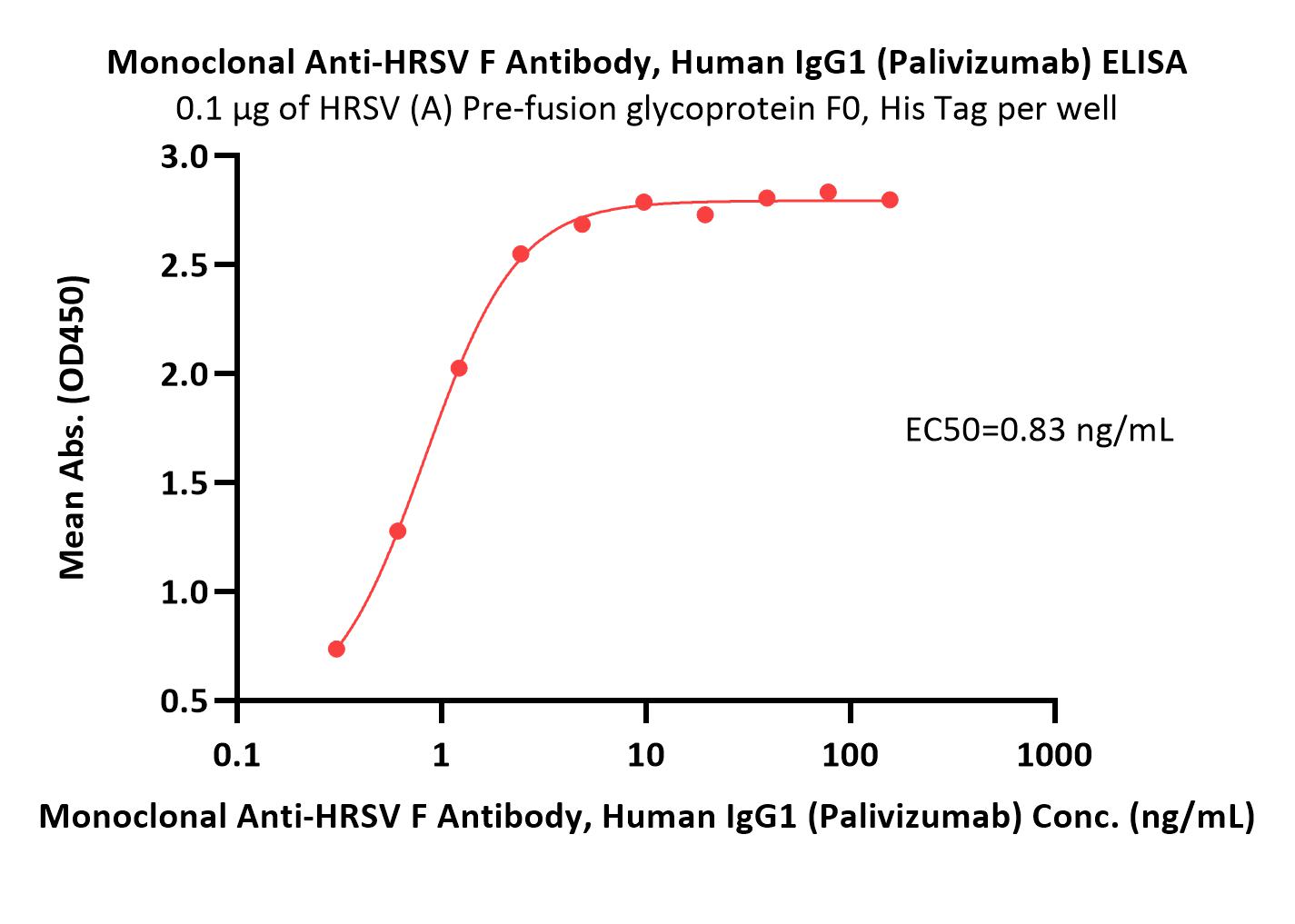























































 膜杰作
膜杰作 Star Staining
Star Staining















