产品参数(Product Specifications)
| Assay Type | Sandwich-ELISA |
| Analyte | Protein L |
| Format | 96T(8×12 strips) |
| Regulatory Status | RUO |
| Sensitivity | <50pg/mL |
| Standard Curve Range | 50 pg/mL-3200 pg/mL |
| Assay Time | 2 hr |
| Suitable Sample Type | For the quantitative determination of recombinant protein L |
| Sample volume | 50uL |
产品概述(Product Overview)
resDetect™ Protein L ELISA Kit is based on the ELISA sandwich method and is used to measure natural or structurally conserved recombinant forms of Protein L, as well as recombinant forms of Protein L with significant structural differences from natural Protein A, such as MabSelect™ VL, in bioprocess manufacturing applications. It is designed to provide a reliable solution to aid in optimal purification process development and routine quality control of in-process streams and final products. It can also be used as a universal detection tool for the quantitative determination of Protein L.
存储(Storage)
1. Unopened kit should be stored at 2℃-8℃ upon receiving.
2. Find the expiration date on the outside packaging and do not use reagents past their expiration date.
3. The opened kit should be stored per components table. The shelf life is 30 days from the date of opening.
组分(Materials Provided)
| ID | Components | Size |
| RES026-C01 | Pre-Coated Anti-Protein L Antibody Microplate | 1 plate(8×12 strips) |
| RES026-C02 | Recombinant Protein L Standard (1μg/mL) | 100μL |
| RES026-C03 | Biotin-Anti-Protein L Antibody | 150uL |
| RES026-C04 | Streptavidin-HRP | 10μg |
| RES026-C05 | 10×Sample Dilution Buffer | 15mL |
| RES026-C06 | Denaturation Buffer | 15mL |
| RES026-C07 | 20×Washing Buffer | 30mL |
| RES026-C08 | Antibody Dilution Buffer | 15mL |
| RES026-C09 | Streptavidin-HRP Dilution Buffer | 15mL |
| RES026-C10 | Substrate Solution | 12mL |
| RES026-C11 | Stop Solution | 8mL |
质量管理控制体系(QMS)
典型数据-Typical Data Please refer to DS document for the assay protocol.
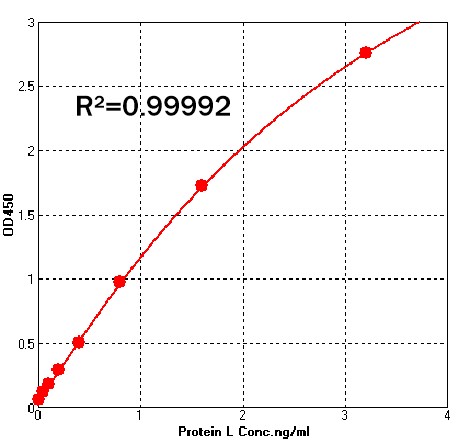
Detection of Recombinant Protein L by sandwich-ELISA Assay. Immobilized Anti- Protein L Antibody can bind Recombinant Protein L . Detection was performed using Biotin-Anti- Protein L Antibody with sensitivity of 50 pg/mL. For each experiment, a standard curve needs to be set for each micro-plate, and the specific OD value may vary depending on different laboratories, testers, or equipments. The following example data is for reference only.
验证(Validation)
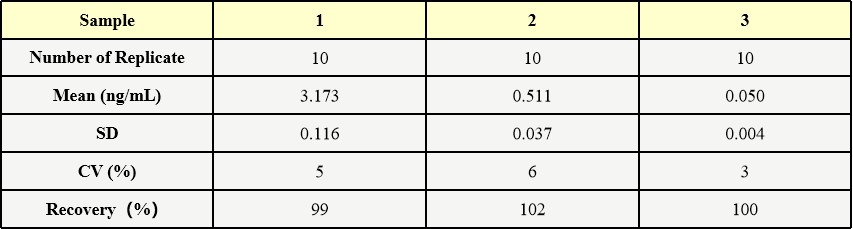
批内差异(Intra-Assay Statistics)
Three samples of known concentration were tested ten times on one plate to assess intra-assay precision.
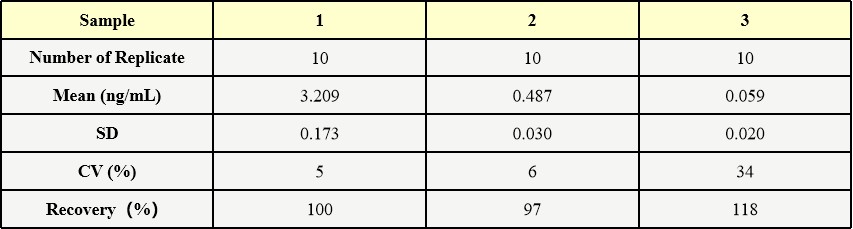
批间差异(Inter-Assay Statistics)
Three samples of known concentration were tested in three separate assays to assess inter-assay precision.
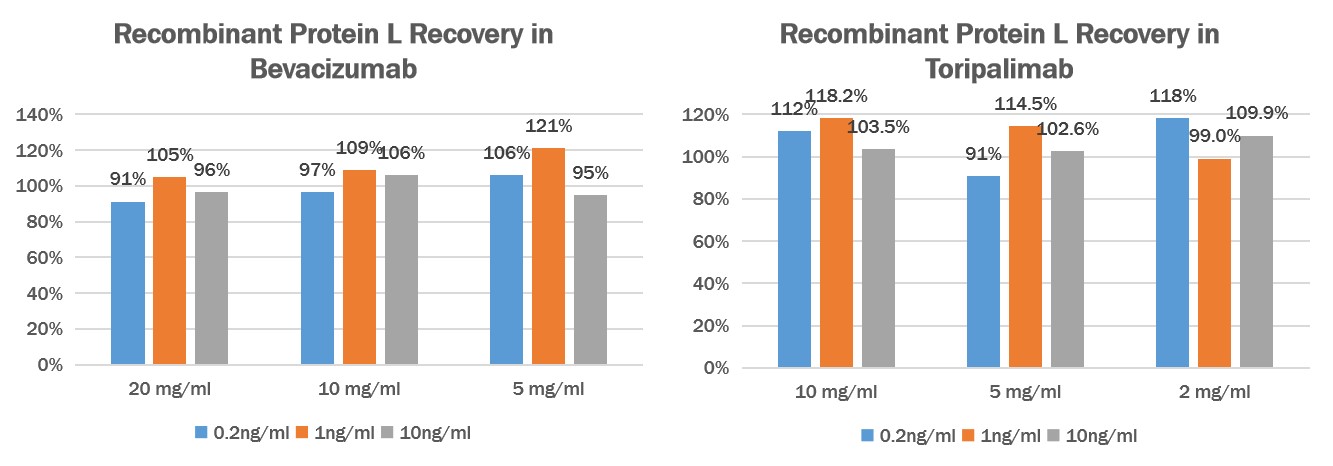
回收率(Recovery)
Add different concentrations of Protein L (0.2ng/mL、1ng/mL、10ng/mL) to different concentrations of Human IgG1 (Bevacizumab) (20mg/mL、10mg/mL、5mg/mL) or Human IgG4 (Toripalimab) (10mg/mL、5mg/mL、2mg/mL), then dilute the antibodies to a reasonable range, then test and calculated the concentration of protein L to give the recovery rate.
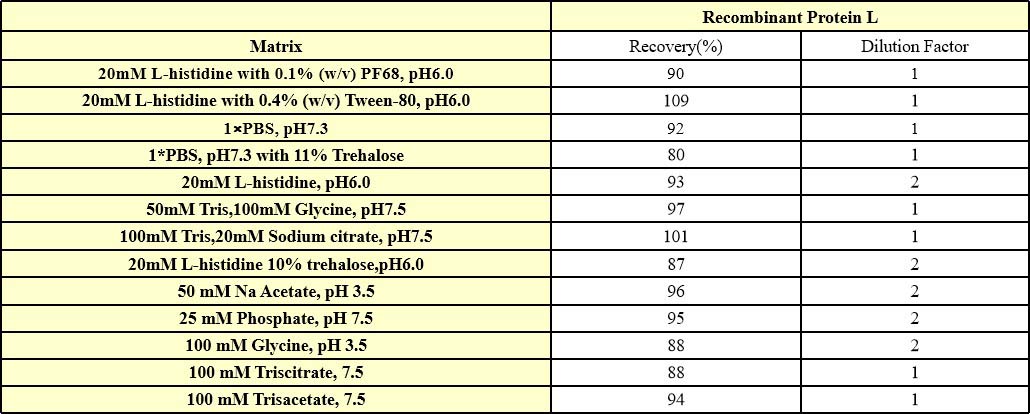
干扰效应(Interference effect)
We have conducted interference effect test about frequently-used buffers, they have excellent buffer compatibility. For specific buffers, it is recommended that you verify recovery to determine the minimum dilution ratio.
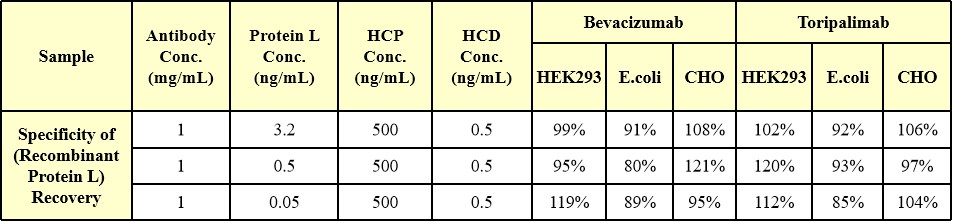
专属性(Specificity)
Host cell protein (HCP 500 ng/mL) and host cell DNA (HCD 0.5 ng/mL) of HEK293, E.coli or CHO systems were added to human IgG1 (Bevacizumab, 1mg/mL) and human IgG4 (Toripalimab, 1mg/mL), respectively, which were higher than the usual quality standard limit. Then high, medium, and low concentrations of Protein L were added, respectively, and the ratio of Protein L recovery in the Protein L added samples without HCP and HCD was added as the specificity verification index. verification index.
背景(Background):protein L
Protein L was isolated from the surface of bacterial species Peptostreptococcus magnus and was found to bind Ig(IgG,IgM,IgA,IgE and IgD) through L chain interaction, from which the name was suggested. Despite this wide-ranging binding capability with respect to Ig classes, Protein L is not a universal immunoglobilin-binding protein. Binding of Protein L to immunoglobulins is restricted to those containing kappa light chains (i.e., k chain of the VL domain). In humans and mice, kappa (k) light chains predominate. The remaining immunoglobulins have lambda (l) light chains. The recombinant protein contains four immunoglobulin (Ig) binding domains (Bdomains) of the native protein. Besides antibody, protein L is also suitable for binding of a wide range of antibody fragments such as Fabs, single-chain variable fragments (scFv), and domain antibodies (Dabs).























































 膜杰作
膜杰作 Star Staining
Star Staining















