分子别名(Synonym)
TNFRSF25,DR3,APO3,DDR3,TNFRSF12,WSL,WSL1
表达区间及表达系统(Source)
Biotinylated Human DR3 Protein, His,Avitag (DR3-H82E3) is expressed from human 293 cells (HEK293). It contains AA Gln 25 - Gln 199 (Accession # Q93038-1).
Request for sequence
蛋白结构(Molecular Characterization)

This protein carries a polyhistidine tag at the C-terminus, followed by an Avi tag (Avitag™).
The protein has a calculated MW of 22.6 kDa. The protein migrates as 30-35 kDa when calibrated against Star Ribbon Pre-stained Protein Marker under reducing (R) condition (SDS-PAGE) due to glycosylation.
标记(Labeling)
Biotinylation of this product is performed using Avitag™ technology. Briefly, the single lysine residue in the Avitag is enzymatically labeled with biotin.
蛋白标记度(Protein Ratio)
Passed as determined by the HABA assay / binding ELISA.
内毒素(Endotoxin)
Less than 1.0 EU per μg by the LAL method.
纯度(Purity)
>90% as determined by SDS-PAGE.
>90% as determined by SEC-MALS.
制剂(Formulation)
Lyophilized from 0.22 μm filtered solution in PBS, pH7.4 with trehalose as protectant.
Contact us for customized product form or formulation.
重构方法(Reconstitution)
Please see Certificate of Analysis for specific instructions.
For best performance, we strongly recommend you to follow the reconstitution protocol provided in the CoA.
存储(Storage)
For long term storage, the product should be stored at lyophilized state at -20°C or lower.
Please avoid repeated freeze-thaw cycles.
This product is stable after storage at:
- -20°C to -70°C for 12 months in lyophilized state;
- -70°C for 3 months under sterile conditions after reconstitution.
质量管理控制体系(QMS)
电泳(SDS-PAGE)
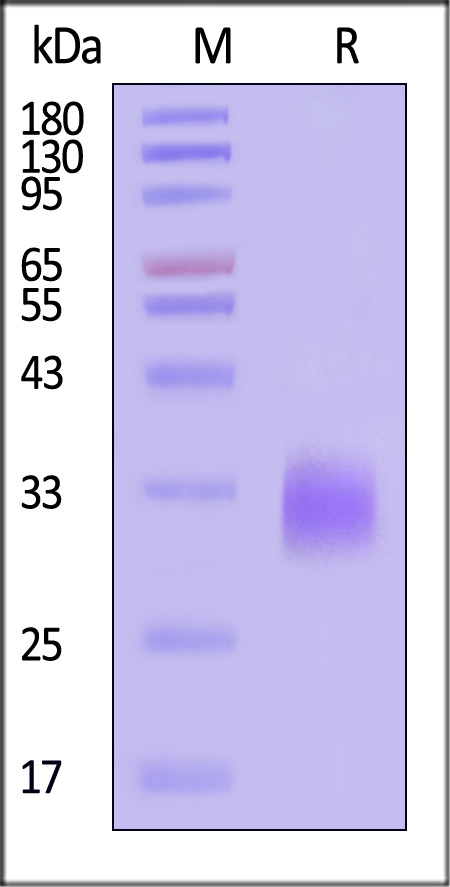
Biotinylated Human DR3 Protein, His,Avitag on SDS-PAGE under reducing (R) condition. The gel was stained with Coomassie Blue. The purity of the protein is greater than 90% (With Star Ribbon Pre-stained Protein Marker).
SEC-MALS
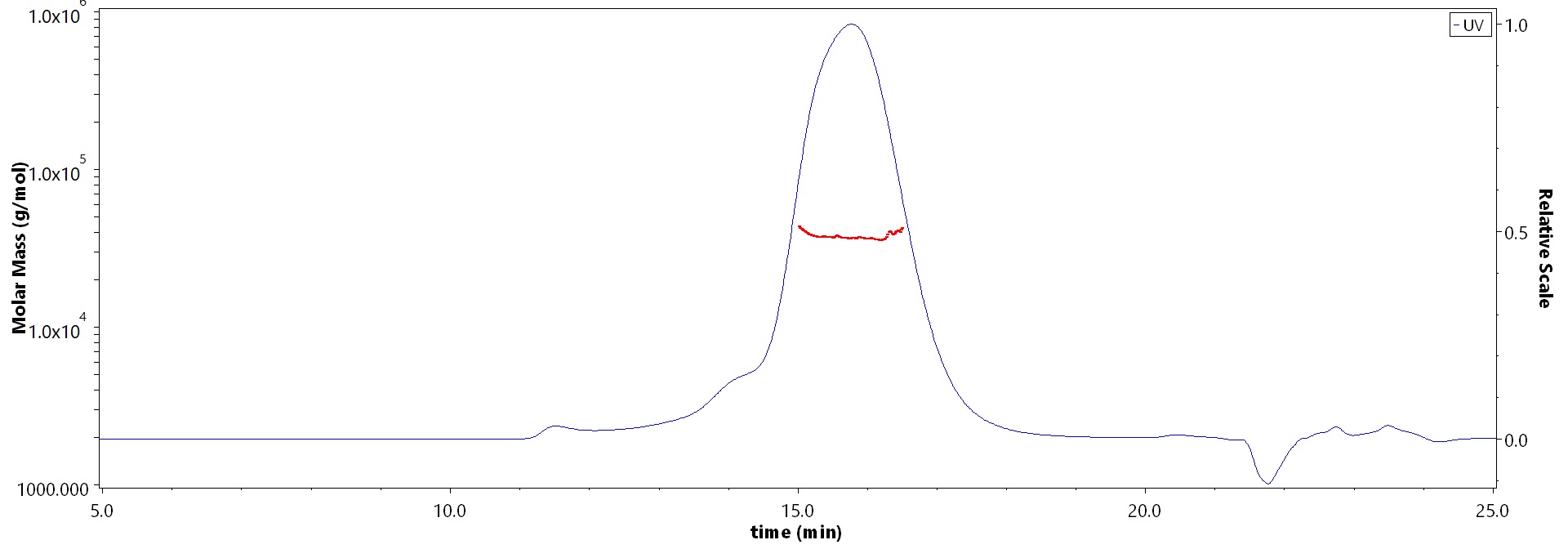
The purity of Biotinylated Human DR3 Protein, His,Avitag (Cat. No. DR3-H82E3) is more than 90% and the molecular weight of this protein is around 30-45 kDa verified by SEC-MALS.
Report
活性(Bioactivity)-ELISA
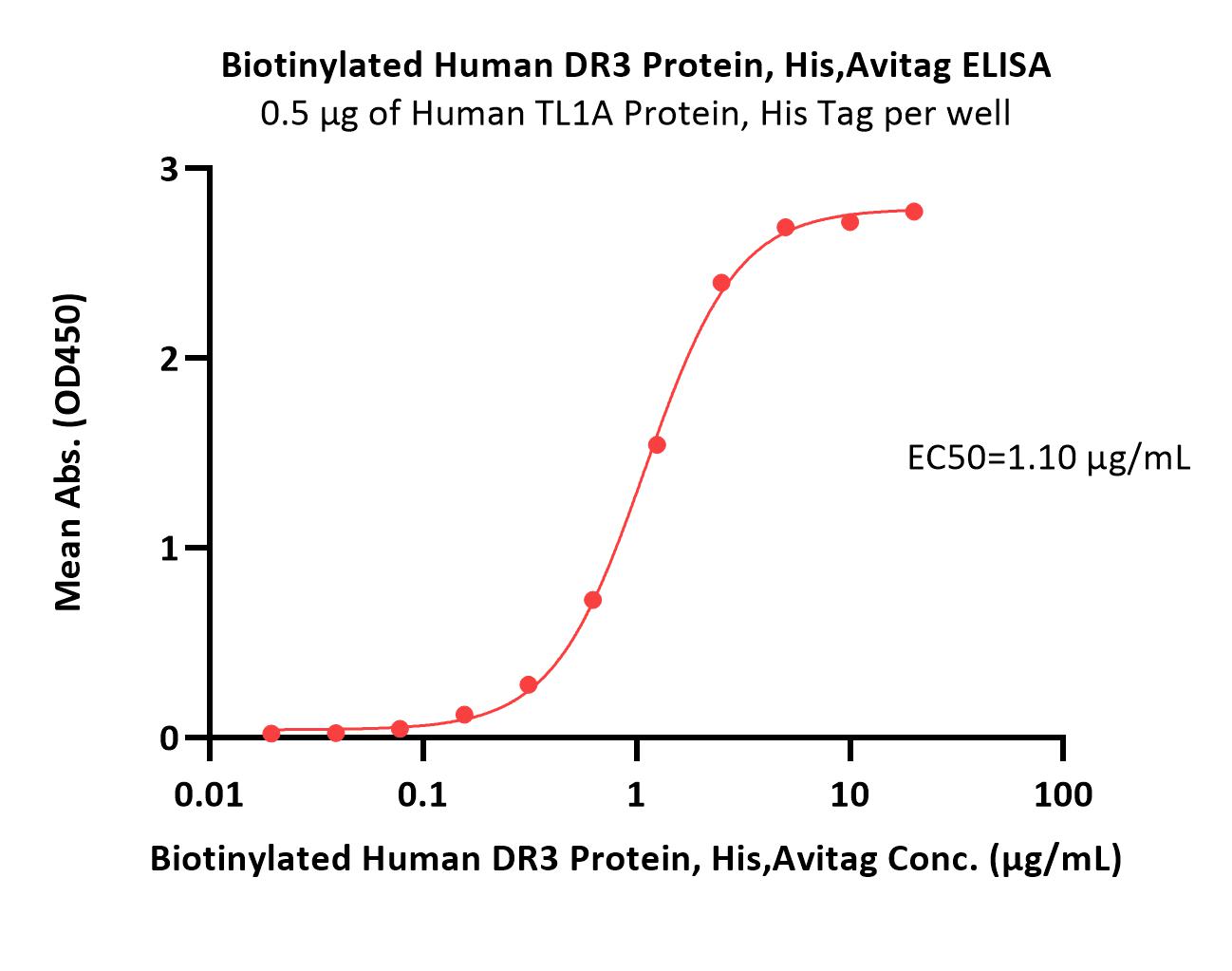
Immobilized Human TL1A Protein, His Tag (Cat. No. TLA-H5244) at 5 μg/mL (100 μL/well) can bind Biotinylated Human DR3 Protein, His,Avitag (Cat. No. DR3-H82E3) with a linear range of 0.02-2.5 μg/mL (QC tested).
Protocol
活性(Bioactivity)-SPR
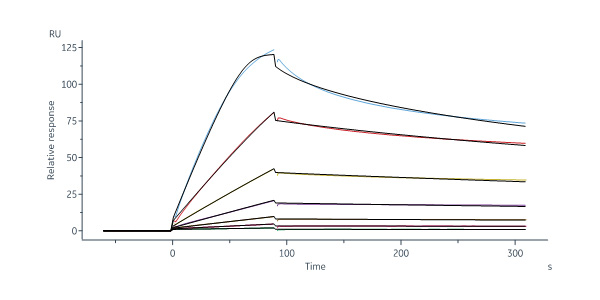
Biotinylated Human DR3 Protein, His,Avitag (Cat. No. DR3-H82E3) captured on Biotin CAP-Series S Sensor Chip can bind Human TL1A, His Tag (Cat. No. TLA-H5243) with an affinity constant of 1.75 nM as determined in a SPR assay (Biacore 8K) (Routinely tested).
Protocol
背景(Background)
Tumor necrosis factor receptor superfamily member 25 (TNFRSF25) is also known as Apo-3, Death receptor 3 (DDR3 or DR3), Apoptosis-inducing receptor AIR, Apoptosis-mediating receptor TRAMP, Lymphocyte-associated receptor of death, Apo-3, which is a member of the TNF-receptor superfamily. TNFRSF25 is a homodimer protein, which can Interact strongly via the death domains with TNFRSF1 and TRADD to activate at least two distinct signaling cascades, apoptosis and NF-kappa-B signaling. TNFRSF25 is receptor for TNFSF12 / APO3L / TWEAK.























































 膜杰作
膜杰作 Star Staining
Star Staining
















